Abstract
Background and purpose:
The mechanism for the development of post-haemorrhagic cerebral vasospasm after subarachnoid haemorrhage (SAH) still remains unknown.
Experimental approach:
We investigated the role of thrombin and its receptor PAR1 in the development of hyper-contractility of the basilar artery in a rabbit double haemorrhage model, which received two injections of autologous blood into the cisterna magna.
Key results:
In the basilar artery isolated from the control rabbits, thrombin, only at 10 units ml−1, induced a transient endothelium-dependent relaxation and a slight smooth muscle contraction. In SAH, the contractile response to thrombin was markedly enhanced, while the endothelium-dependent relaxant effect of thrombin remained unchanged. The enhancement of the contractile responses was also observed in the absence of endothelium and thrombin induced an enhanced contraction at concentrations higher than 0.3 units ml−1. The contractile response to PAR1-activating peptide was also enhanced after SAH. However, the contractile responses to high K+ and endothelin-1, and the myofilament Ca2+-sensitivity remained unchanged after SAH. An immunoblot analysis suggested the up-regulation of PAR1 in the smooth muscle of the basilar artery. The heparinization of blood before injection prevented the enhancement of the contractile responses to thrombin and PAR1-activating peptide.
Conclusions and implications:
The present study demonstrated, for the first time, that the contractile response of the basilar artery to thrombin was markedly enhanced after SAH. Mechanistically, our findings suggested that the activation of thrombin following hemorrhage up-regulated the expression of PAR1, thereby inducing the hyper-responsiveness to thrombin.
Keywords: thrombin, post-haemorrhagic cerebral vasospasm, smooth muscle, basilar artery, rabbit
Introduction
Post-haemorrhagic cerebral vasospasm is a major cause of death and disability in patients with subarachnoid haemorrhage (SAH) (Kassell et al., 1985). Prevention and treatment of vasospasm thus plays an important role in the management of SAH. However, the molecular mechanism for development of vasospasm still remains to be elucidated. The increased production of spasmogens (Macdonald and Weir, 1991; Tosaka et al., 2001) and/or increased vascular responsiveness (Harder et al., 1987; Todo et al., 1998; Sato et al., 2000) is considered to contribute to the development of vasospasm. A clinical study demonstrated that the incidence of post-haemorrhagic vasospasm correlates with the amount of peri-arterial blood clotting (Fisher et al., 1980). The thrombin activity in the cerebrospinal fluid was shown to correlate with the development of vasospasm in SAH patients (Kasuya et al., 1998). An inhibition of the thrombin activity was reported to prevent the development of vasospasm in a rabbit SAH model (Zhang et al., 2001). It is thus conceivable that thrombin plays a crucial role in the development of post-haemorrhagic vasospasm. Thrombin activates proteinase-activated receptor 1, 3 and 4 (PAR1, PAR3 and PAR4) (Coughlin, 2000), and it induces an endothelium-dependent vasorelaxation and contraction of the vascular smooth muscle mainly via PAR1 (Dery et al., 1998; Macfarlane et al., 2001; Hollenberg and Compton, 2002; Hirano and Kanaide, 2003). The thrombin–PAR1 pathway is therefore considered to play a critical role in the development of post-haemorrhagic vasospasm. However, such a possibility still remains to be examined.
In the present study, the role of the thrombin–PAR1 pathway in the development of hyper-contractility after SAH was investigated by using a rabbit double haemorrhage SAH model. We herein report, for the first time, that vascular responsiveness to thrombin was markedly augmented after SAH. Importantly, heparinization of blood before injection prevented the enhancement of contractile response after SAH. The present study thus proposes a novel mechanism involving the thrombin–PAR1 pathway for the development of post-haemorrhagic vasospasm after SAH.
Methods
Preparation of subarachnoid haemorrhage rabbit model
This study protocol was approved by the Committee of Ethics on Animal Experiments in the Graduate School of Medical Sciences, Kyushu University.
Adult male Japanese white rabbits (2.5–3.0 kg) were anaesthetized with an intramuscular injection of ketamine (40 mg kg−1) and an intravenous injection of sodium pentobarbital (20 mg kg−1). A longitudinal, midline suboccipital incision (3.0 cm) was centred over the foramen magnum, and the neck muscles were dissected until the dura was visualized. With the use of a 23-gauge butterfly needle, 0.5 ml of cerebrospinal fluid was aspirated from the cisterna magna, and then 2.5 ml of autologous arterial blood obtained from the middle branch of the ear artery was injected immediately (day 0). The animal was then positioned on an inclined board at a 30° angle with the head down in neutral position for 30 min. The second injection of autologous blood was similarly performed on day 2. The control animals received injections of the same volume of saline. In the experiments with heparinization (Figure 2), heparin was added to the autologous blood (final concentration of 100 U ml−1) before injecting the blood into the cisterna magna.
Ring preparations of basilar artery
Seven days after the first injection of autologous blood, unless otherwise specified, the rabbits were heparinized (1000 U), given an intravenous injection of a lethal dose of sodium pentobarbital (120 mg kg−1) and exsanguinated from the carotid artery. The basilar artery was immediately excised and arterial rings measuring 500 μm in width were prepared. To remove the endothelium, the internal surface was rubbed with a tungsten wire. Carbachol (1 μM) induced relaxation in the preparations with an intact endothelium, but not in those with the endothelium removed (data not shown). The loss of the relaxant effect due to carbachol was thus used to confirm the functional removal of endothelial cells. The ring preparations were kept in normal physiological salt solution (PSS; see below) at room temperature until use.
Measurement of tension in intact ring preparations of basilar artery
The measurement of tension in the intact ring preparations of basilar artery was performed as described previously (Nakamura et al., 2001). In brief, the ring preparations were mounted horizontally between two tungsten wires in an organ bath containing 2 ml buffer. One of the wires was connected to the force transducer U Gauge (Minebea, Nagano, Japan), while the other was fixed. The preparations were equilibrated in normal PSS at 37 °C for at least 60 min before starting the experimental protocol. During the 60-min equilibration period, the rings were stimulated with 118 mM K+ every 15 min, increasing the resting load in a stepwise manner. The optimal resting load was finally adjusted to 50 mg. Under such a resting load, the contractile responses to thrombin and other stimulations were studied. The data were expressed as a percentage, assigning the level of tension obtained in normal PSS and that obtained during the sustained phase of the 118 mM K+-induced contraction at 5 min to be 0 and 100%, respectively, unless otherwise specified.
Measurement of cytosolic Ca2+ concentration in intact ring preparations
The arterial rings were loaded with the Ca2+ indicator dye fura-2, as described previously (Maeda et al., 2003; Kanaide, 2006). The simultaneous measurement of tension and fura-2 fluorescence was performed as described previously (Nakamura et al., 2001; Maeda et al., 2003). The ring preparations were mounted horizontally between two tungsten wires in an organ bath, which was set on the stage of a TMD56 inverted fluorescence microscope (Nikon, Tokyo, Japan) equipped with a CAM-220 fluorometer (JASCO, Tokyo, Japan). The fluorescence intensities obtained with 340 and 380 nm excitation lights and their ratio were continuously recorded. The values of fluorescence ratios obtained in normal PSS and those obtained during the sustained phase of the 118 mM K+-induced contraction at 5 min were 0 and 100%, respectively.
Tension measurement in α-toxin-permeabilized preparations
The arterial rings were permeabilized with 5000 U ml−1 staphylococcal α-toxin in Ca2+-free cytosolic substitution solution (CSS; see below) for 30 min at 25 °C, as described previously (Nakamura et al., 2001). Treated rings were mounted horizontally between two tungsten wires for tension measurement, as described above for the measurement of tension in intact ring preparations. The ring preparations were stretched to twice their resting diameter, and then they were allowed to completely relax in Ca2+-free CSS for 30 min before starting the experimental protocols. The extent of developed tension was expressed as a percentage, while assigning the values obtained in Ca2+-free CSS and 10 μM Ca2+-containing CSS to be 0 and 100%, respectively, unless otherwise specified.
Immunoblot analysis of the expression of PAR1
The basilar artery, with endothelium removed, was homogenized in 50 mM Tris–HCl, pH 7.2, 0.5 M NaCl, 10 mM MgCl2, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 1% Triton X-100, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 10 μM 4-aminidophenylmethane sulphonyl fluoride. Fifteen micrograms of total protein was separated on a 7.5–20% gradient polyacrylamide gel in SDS-polyacrylamide gel electrophoresis, and then subjected to an immunoblot analysis using anti-PAR1 antibody (sc-5605, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immune complex was detected using an enhanced chemiluminescence technique, and the light emission was detected and analysed with a ChemiDoc XRS-J image analysis system (BioRad, Tokyo, Japan). After immunoblot detection, the membranes were stained with naphthol blue black to visualize the band corresponding to actin. The optical density of the PAR1 band was normalized to that of actin.
Solutions
The composition of the normal PSS was (in mM) NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25 and D-glucose 11.5, aerated with a mixture of 5% CO2 and 95% O2, with the resulting pH determined to be 7.4. High K+-PSS (118 mM K+) was prepared by equimolar substitution of NaCl for KCl. The composition of Ca2+-free CSS was (in mM) potassium methanesulphonate 100, Na2ATP 2.2, MgCl2 3.38, EGTA 10 and creatine phosphate 10, and Tris-maleate 20 (pH 6.8). The CSS containing the indicated concentration of free Ca2+ was prepared by adding an appropriate amount of CaCl2, while assuming the Ca2+-EGTA binding constant to be 106 M−1, as described previously (Nakamura et al., 2001).
Data analysis
The data are expressed as the means±s.e.m. One strip obtained from one animal was used for each experiment, and, therefore, the number of experiments indicates the number of animals. Student's t-test was used to determine statistical significance between the two groups and an analysis of variance was used to determine the effects of 118 mM K+, endothelin, thrombin, PAR1-AP, Ca2+ and GTPγS. Values of P<0.05 were considered to be statistically significant.
Reagents
Heparin (Mr 3000) and thrombin (bovine plasma, 1050 U mg−1) were purchased from Sigma (St Louis, MO, USA). High-affinity PAR1-activating peptide (PAR1-AP; Ala-Phe(pF)-Arg-Cha-homoArg-Tyr-NH2) was from NEOSYSTEM (Strasbourg, France). SLIGRL-NH2 (PAR2-AP) was obtained from Bachem (Budendorf, Switzerland). AYPGKF-NH2 (PAR4-AP) and FTLLR-NH2 (inactive control peptide of PAR1-AP) were synthesized by Peptide Synthesis Service, University of Calgary (Calgary, AB, Canada).
Results
Enhancement of the contractile response to PAR1 agonists after SAH
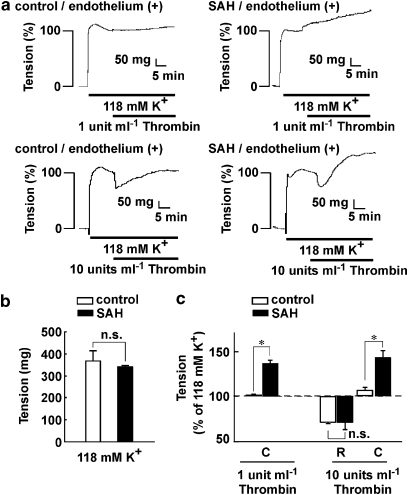
In the presence of endothelium, the tension development in arterial rings induced by 118 mM K+ after SAH was similar to that seen in the control (Figure 1b). The contractile response to 118 mM K+ was thus assigned to be 100%. In the control rings, 1 U ml−1 thrombin did not induce any apparent effect on the 118 mM K+-induced sustained contraction, while 10 U ml−1 thrombin induced a transient relaxation but no apparent contraction (Figures 1a and c). This relaxation was abolished by removing the endothelium (data not shown). In rings prepared after SAH, 1 U ml−1 thrombin induced progressive contraction with no apparent relaxation, while 10 U ml−1 thrombin induced an initial transient relaxation followed by a sustained contraction (Figures 1a and c). The transient relaxation was also abolished by removing the endothelium (data not shown). The extent of endothelium-dependent relaxation seen after SAH was similar to that seen in the control (Figure 1c). The contractile response to thrombin was thus characteristic of arterial rings after SAH.
Figure 1.
Effect of thrombin on tension during contractions induced by 118 mM K+ in rings of the basilar artery with an intact endothelium. (a) Representative recordings showing the effects of 1 and 10 U ml−1 thrombin on the 118 mM K+-induced contraction in the basilar artery with an intact endothelium obtained from control and subarachnoid haemorrhage (SAH) rabbits. (b) The level of tension induced by 118 mM K+ depolarization in the basilar artery with an intact endothelium in control and SAH. The data are the mean±s.e.m. (n=7). n.s., not significantly different. (c) Summary of the maximal relaxation (R) and contraction (C) induced by 1 and 10 U ml−1 thrombin during the 118 mM K+-induced contraction in the basilar artery with an intact endothelium in control and SAH. The level of tension just prior to the application of thrombin during the 118 mM K+-induced contraction was assigned a value of 100%, and it is indicated by a dashed line. The contractile response to thrombin was evaluated 20 min after the application of thrombin. The data are the mean±s.e.m. (n=4). n.s., not significantly different. *P<0.05.
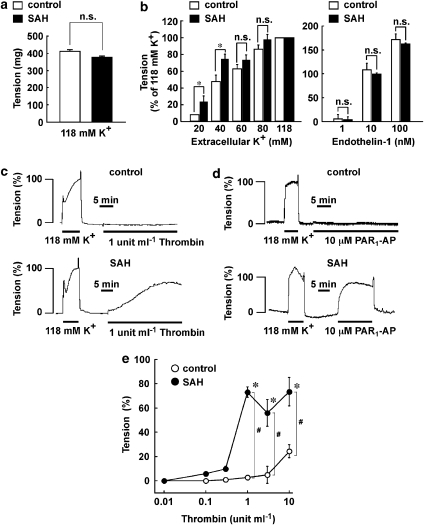
Next, we directly examined the contractile response of smooth muscle in the absence of the endothelium (Figure 2). Under this condition, 118 mM K+ depolarization also induced a sustained contraction, and the extent of tension development seen in the control was similar to that seen after SAH (Figure 2a). Therefore, the contractile response to 118 mM K+ was also assigned to be 100% in the absence of the endothelium. Elevation of the extracellular K+ concentration induced a concentration-dependent contraction, and maximal tension development was obtained at concentrations over 80 mM in both control and SAH rings (Figure 2b). The tension development seen with 60 and 80 mM K+ as well as endothelin-1 (1–100 μM) did not significantly differ between control and SAH, while that seen with 20 and 40 mM K+ after SAH was significantly greater than that in the control (Figure 2b). On the other hand, 1 U ml−1 thrombin induced no apparent contraction in the control artery (Figure 2c). However, it induced a large sustained contraction after SAH, which progressively reached a peak at 30 min (Figure 2c). The analysis of concentration–response curves revealed that thrombin induced a small but significant contraction even in the control, but only at 10 U ml−1 (Figure 2e). However, after SAH, thrombin induced a significant contraction at concentrations higher than 0.3 U ml−1 (Figure 2e). The contractile responses to 1 U ml−1 and higher concentrations of thrombin after SAH were significantly augmented (Figure 2e). PAR1-AP (10 μM) failed to induce any contraction in the control, while it induced a substantial contraction after SAH (Figure 2d). An inactive control peptide for PAR1-AP (FTLLR-NH2), a PAR2-AP (SLIGRL-NH2) and a PAR4-AP (AYPGKF-NH2) induced no contractile response after SAH (data not shown).
Figure 2.
Enhanced contractile response to PAR1 agonists in the basilar artery, without endothelium after subarachnoid haemorrhage (SAH). (a, b) The level of tension induced by high K+ depolarization and endothelin-1 in the basilar artery of control and SAH, with the endothelium removed. The data are the mean±s.e.m. (n=15 in (a); n=3 for control and n=4 for SAH in (b)). *P<0.05, n.s., not significantly different. (c, d) Representative recordings of contractions induced by 1 U ml−1 thrombin (c) and 10 μM PAR1-AP (d) in the basilar artery of rabbits injected with saline (control) or autologous blood (SAH). (e) The concentration–response curves for the thrombin-induced tension development in control and SAH. The data are the mean±s.e.m. (n=4–5). * and #P<0.05 vs the resting level and the control, respectively.
Preventive effect of heparin on the enhancement of contractile responses to thrombin and PAR1-AP after SAH
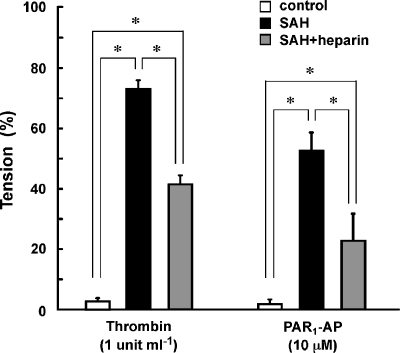
Since the thrombin activity has been shown to correlate with the development of vasospasm after SAH (Kasuya et al., 1998), we hypothesized that the thrombin activity of the injected blood may be responsible for the development of hyper-contractile responses to thrombin and PAR1-AP. We thus examined the effect of injection of heparinized blood on the development of hyper-contractility. In the basilar artery isolated from rabbits that received two injections of autologous blood containing 100 U ml−1 heparin (SAH+heparin), thrombin induced an attenuated contraction compared to that seen after SAH (Figure 3). Similarly, PAR1-AP also induced a significantly attenuated contraction in SAH+heparin (Figure 3).
Figure 3.
Preventive effect of heparinization of autologous blood on the contractile response to thrombin. Summary of the contractile response to 1 U ml−1 thrombin and 10 μM PAR1-AP in the basilar artery of rabbits injected with saline (control) or autologous blood, without (subarachnoid haemorrhage (SAH)) or with heparinization (SAH+heparin), before injection. The contractile response was evaluated in the absence of endothelium. The data are the mean±s.e.m. (n=4–5). *P<0.05.
Contractile response in α-toxin permeabilized preparations
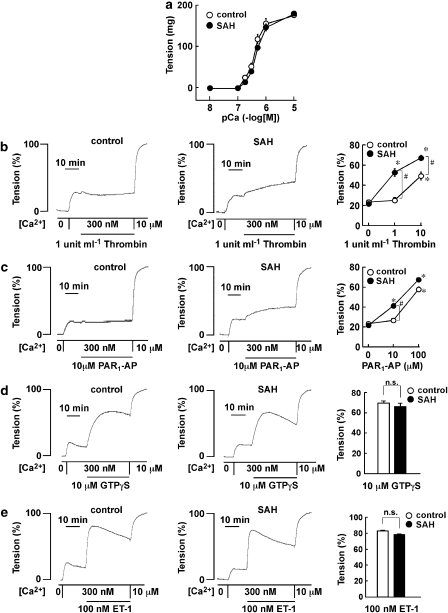
We investigated any possible alteration to the Ca2+-dependent contractile mechanism or the Ca2+ sensitivity of the contractile apparatus after SAH, by using α-toxin permeabilized preparations. A stepwise increase in Ca2+ concentrations induced a similar stepwise development of tension in control and SAH rings, reaching maximal tension development at 10 μM Ca2+ (Figure 4a). The levels of tension obtained at the rest and the sustained contraction induced by 10 μM Ca2+ are thus assigned to be 0 and 100%, respectively. After SAH, 1 U ml−1 thrombin and 10 μM PAR1-AP induced a significant contraction during the 300 nM Ca2+-induced contraction, and elevated the level of tension (Figures 4b and c). In the control, 1 U ml−1 thrombin and 10 μM PAR1-AP had no significant contractile effect (Figures 4b and c). However, the higher concentration of thrombin (10 U ml−1) and PAR1-AP (100 μM) induced significant contractions even in the control, but these contractions were significantly lower than those seen after SAH (Figures 4b and c). On the other hand, the application of 10 μM GTPγS or 100 nM endothelin-1 during the 300 nM Ca2+-induced sustained contraction induced a further development of tension and these contractile effects were similar in rings from control animals and those after SAH (Figures 4d and e). GTPγS was used to directly stimulate the G proteins, thereby increasing the Ca2+ sensitivity of the contractile apparatus (Smigel et al., 1984; Nishimura et al., 1988).
Figure 4.
Contractile responses in rings of rabbit basilar artery, without the endothelium and permeabilized by α-toxin. (a) The pCa2+-tension curves of the contraction induced by stepwise increment of Ca2+ concentrations in α-toxin-permeabilized rabbit basilar artery of control and subarachnoid haemorrhage (SAH) animals. The extent of tension development was expressed as absolute value. The data are the mean±s.e.m. (n=4–5). (b–e) Representative recordings and summary of tension development induced by 1 U ml−1 thrombin (b), 10 μM PAR1-AP (c), 10 μM GTPγS (d) and 100 nM endothelin-1 (e) during contraction induced by 300 nM Ca2+ in control and SAH. The contractile response was evaluated at maximal tension development or at 30 min after induction of precontraction by 300 nM Ca2+. The data are the mean±s.e.m. (n=4–5). *P<0.05 compared to that seen with no addition of thrombin or PAR1-AP; #P<0.05.
Upregulation of the PAR1 expression
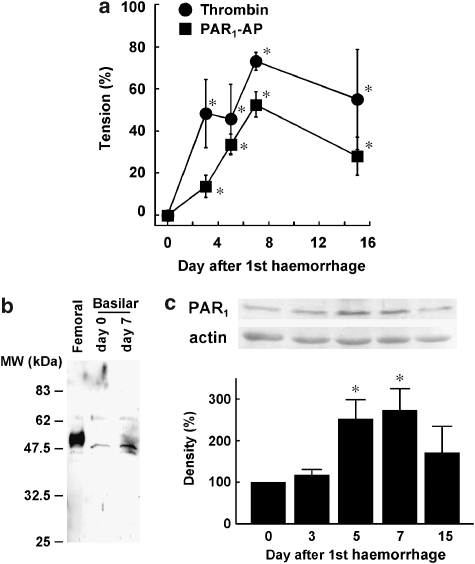
The contractile responses to thrombin and PAR1-AP were augmented depending on the day after SAH, reaching a maximum on day 7 (Figure 5a). Thereafter, the contractile responses were slightly attenuated in comparison to those seen on day 7. However, a significant augmentation of contractile responses was still observed on day 15 (Figure 5a). This time course is consistent with that seen with the development of post-haemorrhagic vasospasm in SAH patients (Cook, 1995). The change in the expression of PAR1 after SAH was evaluated with an immunoblot analysis (Figures 5b and c). We used a rabbit femoral artery obtained 1 week after balloon injury as a positive control, as PAR1 was markedly upregulated after balloon injury (Fukunaga et al., 2006). In the femoral artery, one major band of ∼50 kDa was detected, while a minor band of ∼62 kDa was also detected (Figure 5b). In the basilar artery, a single major band of ∼48 kDa as well as a minor ∼62-kDa band, which is similar to that seen in the femoral artery, was detected. The major band was thus considered to represent PAR1 in the basilar artery, although a slight difference in molecular weight was noted between the femoral and basilar arteries. The density of this band seen on day 7 appeared to be higher than that seen on day 0 (Figure 5b). We thus performed a quantitative evaluation of this band and found that the level of PAR1 expression was significantly increased on days 5 and 7 (Figure 5b). The level of PAR1 seen on day 15 was less than that seen on day 7 (Figure 5c). We also attempted to evaluate the changes in the mRNA level. However, the yield of total RNA from the basilar artery was too low to perform quantitative evaluation of mRNA. In contrast, mRNA for PAR1 was successfully evaluated in samples of rabbit liver (data not shown).
Figure 5.
Time course of enhancement of contractile responses and changes in PAR1 expression in the basilar artery without the endothelium. (a) The changes in the maximal tension developed by 1 U ml−1 thrombin and 10 μM PAR1-AP over 15 days after the initial injection of autologous blood. *P<0.05 vs day 0. (b), Representative immunoblot of PAR1 in rabbit femoral artery obtained 1 week after balloon injury as reported (Fukunaga et al., 2006) and in rabbit basilar artery obtained on days 0 and 7 after subarachnoid haemorrhage (SAH). The molecular weight is indicated on the left. (c) Immunoblot analysis of the change in the expression of PAR1 protein in the basilar artery after SAH. The level of expression seen on day 0 was assigned to be 100%. The data are the mean±s.e.m. (n=4–5 for (a), n=3 for (c), *P<0.05 vs day 0.
The simultaneous measurement of cytosolic Ca2+ concentrations and tension
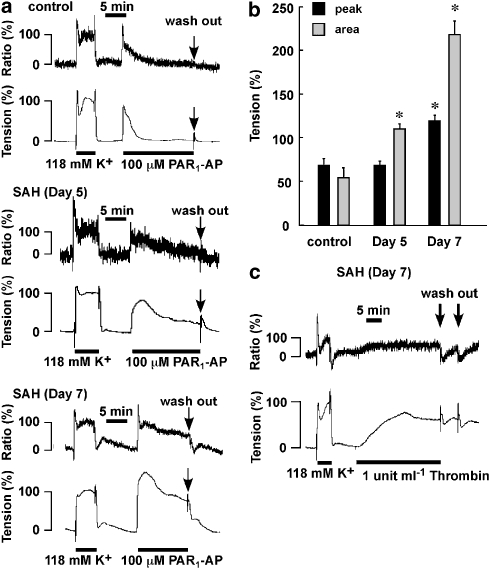
Although 10 μM PAR1-AP failed to induce an apparent contractile response in the control arteries (Figure 2), 100 μM PAR1-AP induced a transient increase in both [Ca2+]i and tension in the control (Figure 6a). After SAH, both the extent and the duration of the PAR1-AP-induced contraction progressively increased with time after the initial injection of autologous blood, resulting in the conversion of a transient contraction in the control to a more sustained contraction after SAH (Figure 6a). The sustained contraction was associated with a sustained increase in [Ca2+]i. Such a conversion of the contractile response to PAR1-AP was supported by the observation that the degree of enhancement of the contraction as evaluated by the area under the tension trace (4.0-fold increase) was greater than that evaluated by the peak tension development (1.7-fold increase) (Figure 6b). Terminating the PAR1-AP stimulation by washing it out during the sustained phase of the contraction caused the tension to revert to a resting level (Figure 6a). On the other hand, the sustained contraction induced by 1 U ml−1 thrombin persisted even after removing the thrombin, in rings after SAH (Figure 6c). In contrast, in the control, the contraction induced by 10 U ml−1 thrombin gradually declined even in the continuous presence of thrombin (data not shown).
Figure 6.
The simultaneous measurement of cytosolic Ca2+ concentrations and tension in the basilar artery without the endothelium. (a) Representative recordings of [Ca2+]i and tension induced by 100 μM PAR1-AP in the basilar arteries in control and subarachnoid haemorrhage (SAH) rabbits (days 5 and 7). (b) The contractile response to 100 μM PAR1-AP was evaluated by the maximum tension development (peak) and the area under the tension trace for the initial 10 min (area), while assigning the values obtained with 118 mM K+-induced contraction to be 100%. The data are the mean±s.e.m. *P<0.05 vs the control. (c) Representative recordings of contraction induced by 1 U ml−1 thrombin in rings taken 7 days after SAH.
Discussion and conclusions
The present study demonstrated, for the first time, that SAH induced a hyper-contractile response to thrombin in a rabbit double haemorrhage model. A thrombin-induced endothelium-dependent relaxation was similarly observed in control arterial rings and those taken after SAH. However, the contractile responses to thrombin and PAR1-AP were substantially augmented after SAH both in the presence and absence of an endothelium. These observations suggest that the augmented contractile response could be attributed to the enhancement of the responsiveness of smooth muscle in SAH, but not to endothelial denudation or dysfunction. The observations with the activating peptides of PAR1, PAR2 and PAR4 suggested that thrombin contracted the basilar artery rings mainly by activating PAR1. On the other hand, the observations with other contractile stimulations (high K+ depolarization, endothelin-1 and GTPγS) in both intact and permeabilized preparations ruled out the general augmentation of the contractile mechanism or any changes in the myofilament Ca2+ sensitivity after SAH. As a result, the hyper-contractile response to thrombin seen after SAH was suggested to be mainly due to enhancement of the function of PAR1 in smooth muscle.
The immunoblot analysis detected one major band of ∼48 kDa in the basilar artery, as well as a minor band of ∼62 kDa. Since the band of ∼48 kDa was the major band noted by inspection of the wide range of molecular weight, and its molecular size was not far from the estimated value based on the amino-acid sequences of human, rat and mouse PAR1, we took this band to represent PAR1 in the rabbit basilar artery. Accordingly, our observations suggest that PAR1 was upregulated after SAH and that this receptor upregulation played a major role in the enhancement of the contractile response to thrombin seen after SAH. However, this conclusion still needs some additional support. First, the specificity of the antibody used in the present study remains to be established, although only one major band was detected in both the basilar and femoral arteries. The detection of one major band may be inconsistent with the previous reports on the existence of glycosylated forms of PAR1, for example by Leger et al. (2006). Second, changes, if any, in the expression of the PAR1 mRNA still remain to be clarified.
There was some discrepancy in the time course between enhancement of the contractile response and PAR1 upregulation. This discrepancy may be due to the difference in the sensitivity of the assay for PAR1 expression. The sensitivity of the immunoblot assay is considered to be lower than that of the bioassay, with the contractile response as an indication of the functional expression of PAR1. However, it is also possible that the PAR1 upregulation may not account for all the mechanism of the enhanced response to thrombin, and this possibility remains to be evaluated.
Under physiological conditions, PAR1 is mainly expressed in vascular endothelial cells, while its expression in smooth muscle is limited (Hirano, 2007). However, the expression of PAR1 in smooth muscle has been shown to be upregulated in vascular lesions such as those seen in atherosclerosis (Nelken et al., 1992; Ku and Dai, 1997) and balloon injury models (Wilcox et al., 1994; Fukunaga et al., 2006). Such upregulation of PAR1 expression in smooth muscle is thus considered to play a critical step in the development of vascular lesions and hyper-contractile state (Hirano, 2007). In this respect, the present study provides the first evidence that the expression of PAR1 was upregulated after SAH. Our observation of the preventive effect of heparin suggested the activation of thrombin or other heparin-sensitive proteinases following haemorrhage to be responsible for the enhancement of responsiveness to thrombin after SAH. As a result, we propose that the production of thrombin after SAH plays a critical role in upregulating the expression of PAR1 in smooth muscle cells, thereby inducing PAR1-mediated hyper-contraction in SAH. The proposed mechanism for PAR1 upregulation is consistent with a previous report showing that thrombin upregulated the expression of PAR1 in endothelial cells (Ellis et al., 1999).
The coagulation cascade was reported to be activated on the onset of SAH (Vermeulen et al., 1985; Kasuya et al., 1988) and higher thrombin activity was detected in the cerebrospinal fluid of patients who developed vasospasm, than in those without vasospasm (Kasuya et al., 1998; Suzuki et al., 1999). Proteinase inhibitors including a thrombin inhibitor, argatroban, were reported to reduce the incidence of post-haemorrhagic vasospasm and to attenuate its severity (Yanamoto et al., 1992; Zhang et al., 2001). All these reports support the critical role of thrombin in the pathogenesis of post-haemorrhagic cerebral vasospasm after SAH. It is thus possible that the thrombin-induced upregulation of PAR1 and the resultant hyper-responsiveness to thrombin, as suggested in the present study, may play a key role in the development of post-haemorrhagic vasospasm. However, this possibility still remains to be evaluated in SAH patients.
We found that the contractile response to PAR1-AP was converted from a transient response in the control to a sustained response in SAH. The sustained contractile response was associated with a sustained elevation of [Ca2+]i. Such sustained PAR1 signalling after SAH may be due to the impairment of desensitization of PAR1. As PAR1 is proteolytically, that is irreversibly, activated, receptor desensitization and endocytosis are considered to play an important role in terminating PAR1 signalling (Brass, 1992; Inglese et al., 1993; Brass et al., 1994; Macfarlane et al., 2001; Hollenberg and Compton, 2002). Otherwise, the proteolytically activated PAR1 would irreversibly elicit a cellular response. Our observations that thrombin induced a sustained contraction in arterial rings after SAH, which persisted even after removal of thrombin, may also support the impairment of receptor desensitization after SAH. Although receptor upregulation plays a major role in the augmentation of PAR1 function after SAH, impairment of receptor desensitization may play a supplemental role.
It should be noted that the inhibitory effect of heparin on the enhancement of contractile response to thrombin and PAR1-AP was still partial. Since the heparinized blood (containing 100 U ml−1 heparin) can be assumed to be almost completely devoid of the activity of thrombin and other heparin-sensitive proteinases, the heparin-sensitive proteinases could not account for all mechanisms for the induction of hyper-responsiveness. Known spasmogens such as platelet products (Yanamoto et al., 1992; Tosaka et al., 2001) and free radicals (Macdonald and Weir, 1991) could be other factors contributing to induction of the upregulation of PAR1 and hyper-contractile responsiveness. Such factors, however, remain to be identified.
In conclusion, we herein demonstrated for the first time that a cerebral artery exhibited hyper-responsiveness to PAR1 agonists after SAH. The enhanced responsiveness was suggested to be mainly due to the upregulation of PAR1 expression in smooth muscle cells, while impairment of receptor desensitization also played a supplemental role. Our observations suggested that the production of thrombin following haemorrhage plays a key role in the upregulation of PAR1 and the enhanced contractile response to thrombin. Thrombin has been suggested to play a critical role in the pathogenesis of post-haemorrhagic cerebral vasospasm after SAH. The present study thus suggests a novel mechanism involving the thrombin–PAR1 pathway for development of vasospasm after SAH. Our proposal, however, remains to be investigated in SAH patients.
Acknowledgments
We thank Mr Brian Quinn for linguistic help with the manuscript. This study was supported in part by grants from the 21st Century COE Program, Grants-in-Aids for Scientific Research (nos. 17590744, 18209045, 18791022) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Research for Promoting Technological Seeds grant from Japan Science and Technology Agency.
Abbreviations
- CSS
cytosolic substitution solution
- PAR
proteinase-activated receptor
- PAR1-AP
PAR1-activating peptide
- PAR2-AP
PAR2-activating peptide
- PAR4-AP
PAR4-activating peptide
- PSS
physiological salt solution
- SAH
subarachnoid haemorrhage
- SDS
sodium dodecyl sulphate
Conflict of interest
The authors state no conflict of interest.
References
- Brass LF. Homologous desensitization of HEL cell thrombin receptors. Distinguishable roles for proteolysis and phosphorylation. J Biol Chem. 1992;267:6044–6050. [PubMed] [Google Scholar]
- Brass LF, Pizarro S, Ahuja M, Belmonte E, Blanchard N, Stadel JM, et al. Changes in the structure and function of the human thrombin receptor during receptor activation, internalization, and recycling. J Biol Chem. 1994;269:2943–2952. [PubMed] [Google Scholar]
- Cook DA. Mechanisms of cerebral vasospasm in subarachnoid haemorrhage. Pharmacol Ther. 1995;66:259–284. doi: 10.1016/0163-7258(94)00080-m. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Ellis CA, Malik AB, Gilchrist A, Hamm H, Sandoval R, Voyno-Yasenetskaya T, et al. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J Biol Chem. 1999;274:13718–13727. doi: 10.1074/jbc.274.19.13718. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurg. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Hirano K, Hirano M, Niiro N, Nishimura J, Maehara Y, et al. The up-regulation of proteinase-activated receptors and the hyper-contractile responses precede the development of arterial lesions after balloon injury. Am J Physiol Heart Circ Physiol. 2006;291:H2388–H2395. doi: 10.1152/ajpheart.01313.2005. [DOI] [PubMed] [Google Scholar]
- Harder DR, Dernbach P, Waters A. Possible cellular mechanism for cerebral vasospasm after experimental subarachnoid hemorrhage in the dog. J Clin Invest. 1987;80:875–880. doi: 10.1172/JCI113146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K. The roles of proteinase-activated receptors in the vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:27–36. doi: 10.1161/01.ATV.0000251995.73307.2d. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kanaide H. Role of protease-activated receptors in the vascular system. J Atheroscler Thromb. 2003;10:211–225. doi: 10.5551/jat.10.211. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. International union of pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ. Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- Kanaide H.Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorometry Methods Mol Biol 2006Humana Press: Totowa, NJ; 251–259.In: Lambert DG (ed) [PubMed] [Google Scholar]
- Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Shimizu T, Okada T, Takahashi K, Summerville T, Kitamura K. Activation of the coagulation system in the subarachnoid space after subarachnoid haemorrhage: serial measurement of fibrinopeptide A and bradykinin of cerebrospinal fluid and plasma in patients with subarachnoid haemorrhage. Acta Neurochir. 1988;91:120–125. doi: 10.1007/BF01424566. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Shimizu T, Takakura K. Thrombin activity in CSF after SAH is correlated with the degree of SAH the persistence of subarachnoid clot and the development of vasospasm. Acta Neurochir. 1998;140:579–584. doi: 10.1007/s007010050143. [DOI] [PubMed] [Google Scholar]
- Ku DD, Dai J. Expression of thrombin receptors in human atherosclerotic coronary arteries leads to an exaggerated vasoconstrictory response in vitro. J Cardiovasc Pharmacol. 1997;30:649–657. doi: 10.1097/00005344-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, et al. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Maeda Y, Hirano K, Nishimura J, Sasaki T, Kanaide H. Rho-kinase inhibitor inhibits both myosin phosphorylation-dependent and -independent enhancement of myofilament Ca2+ sensitivity in the bovine middle cerebral artery. Br J Pharmacol. 2003;140:871–880. doi: 10.1038/sj.bjp.0705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nishimura J, Hirano K, Ibayashi S, Fujishima M, Kanaide H. Hydroxyfasudil, an active metabolite of fasudil hydrochloride, relaxes the rabbit basilar artery by disinhibition of myosin light chain phosphatase. J Cereb Blood Flow Metab. 2001;21:876–885. doi: 10.1097/00004647-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Nelken NA, Soifer SJ, O'Keefe J, Vu TK, Charo IF, Coughlin SR. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992;90:1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Kolber M, van Breemen C. Norepinephrine and GTP-γ-S increase myofilament Ca2+ sensitivity in α-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- Smigel M, Katada T, Northup JK, Bokoch GM, Ui M, Gilman AG. Mechanisms of guanine nucleotide-mediated regulation of adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:1–18. [PubMed] [Google Scholar]
- Suzuki M, Kudo A, Otawara Y, Hirashima Y, Takaku A, Ogawa A. Extrinsic pathway of blood coagulation and thrombin in the cerebrospinal fluid after subarachnoid hemorrhage. Neurosurg. 1999;44:487–493. doi: 10.1097/00006123-199903000-00029. [DOI] [PubMed] [Google Scholar]
- Todo H, Ohta S, Wang J, Ichikawa H, Ohue S, Kumon Y, et al. Impairment in biochemical level of arterial dilative capability of a cyclic nucleotides-dependent pathway by induced vasospasm in the canine basilar artery. J Cereb Blood Flow Metab. 1998;18:808–817. doi: 10.1097/00004647-199807000-00011. [DOI] [PubMed] [Google Scholar]
- Tosaka M, Okajima F, Hashiba Y, Saito N, Nagano T, Watanabe T, et al. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo—possible role in pathogenesis of cerebral vasospasm. Stroke. 2001;32:2913–2919. doi: 10.1161/hs1201.099525. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, van Vliet HH, Lindsay KW, Hijdra A, van Gijn J. Source of fibrin/fibrinogen degradation products in the CSF after subarachnoid hemorrhage. J Neurosurg. 1985;63:573–577. doi: 10.3171/jns.1985.63.4.0573. [DOI] [PubMed] [Google Scholar]
- Wilcox J, Rodriguez J, Subramanian R, Ollerenshaw J, Zhong C, Hayzer D, et al. Characterization of thrombin receptor expression during vascular lesion formation. Circ Res. 1994;75:1029–1038. doi: 10.1161/01.res.75.6.1029. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Kikuchi H, Sato M, Shimizu Y, Yoneda S, Okamoto S. Therapeutic trial of cerebral vasospasm with the serine protease inhibitor, FUT-175, administered in the acute stage after subarachnoid hemorrhage. Neurosurg. 1992;30:358–363. doi: 10.1227/00006123-199203000-00008. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nagata I, Kikuchi H, Xue JH, Sakai N, Sakai H, et al. Broad-spectrum and selective serine protease inhibitors prevent expression of platelet-derived growth factor-BB and cerebral vasospasm after subarachnoid hemorrhage—vasospasm caused by cisternal injection of recombinant platelet-derived growth factor-BB. Stroke. 2001;32:1665–1672. doi: 10.1161/01.str.32.7.1665. [DOI] [PubMed] [Google Scholar]