ABSTRACT
Meningoencephaloceles are herniations of brain tissue through dehiscences of the skull base. These skull defects are either acquired (otologic infection, trauma, surgery, neoplasia) or spontaneous. Spontaneous temporal bone meningoencephaloceles are quite rare conditions, usually congenital in origin presenting during childhood, and only occasionally idiopathic presenting during adulthood. We present a case of temporal bone meningoencephalocele of adult onset. The patient was treated with exploratory mastoidectomy, amputation of the herniated cele and closure of the defect with temporalis fascia and an inferiorly based pedicled muscular flap. No reconstruction of the bony defect was performed, as the layered closure was considered adequate. Twelve months' follow-up revealed no relapse of the condition or postoperative complications.
Keywords: Temporal bone, meningoencephalocele, brain herniation
Meningoencephaloceles (MECs) are herniations of brain tissue, covered by meninges, via openings of the skull base. These bony dehiscences—and the resulting MECs—can be caused by various processes such as otologic infection, trauma, surgery, neoplasia, or may occur spontaneously (Table 1). Chronic otitis media and/or middle ear surgery are considered the main causative factor of MECs; Jackson et al1 reported a series of 35 brain herniation cases, 88.6% of which were secondary to otologic infection and/or surgery. Spontaneous MECs are quite rare, with an incidence of about 8.6% among MECs.2 Spontaneous MECs are either congenital in origin or are otherwise considered idiopathic presenting during adulthood.2,3,4,5,6,7,8,9 In idiopathic cases they are usually located at the tegmen tympani or the tegmen mastoideum. We present an unusual case of tegmen tympani meningoencephalocele, which was occult until adulthood.
Table 1.
Classification of Skull Base Defects
| Spontaneous |
| • Congenital |
| • Idiopathic |
| Acquired |
| • Nontraumatic |
| Chronic otitis media |
| With cholesteatoma |
| Without cholesteatoma |
| Neoplastic |
| Miscellaneous |
| • Iatrogenic |
| Surgical |
| Irradiation |
| • Traumatic (blunt, penetrating) |
CASE REPORT
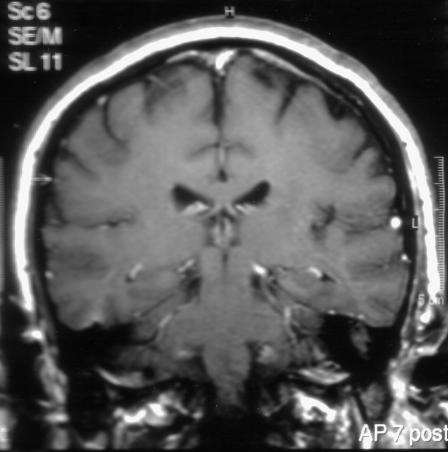
A 49-year-old male patient referred by his physician presented at the ENT clinic with a history of intermittent episodes of left otorrhea, with no other associated symptoms. As part of his medical history the patient reported two episodes of meningitis, requiring admission to the intensive care unit. The bacteria isolated were Streptococcus pneumonia in his first admission and Haemophilus influenza in the second. The brain magnetic resonance image (MRI) in the second admission reported increased signal of the dura in both frontal and parietal regions and a soft-tissue signal in the area of the left epitympanum on T1 sequences (Fig. 1). The temporal bone CT scan commented on inflammatory elements in the left middle ear, with no ossicular destruction and decreased pneumatization of the left mastoid air cell system.
Figure 1.
Coronal T1-weighted magnetic resonance imaging scan of the temporal bone showing a soft-tissue mass in the region of the left temporal bone.
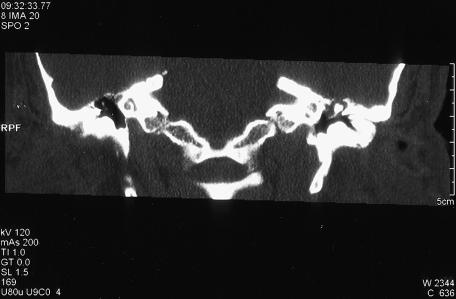
On admission to our department, otoscopy revealed a clear-fluid discharge from the left ear. Examination of the external auditory canal was difficult due to multiple osteomata, which obscured the tympanic membrane. Pure-tone audiometry revealed conductive hearing loss. A high-resolution (HR) helical CT scan revealed a soft-tissue mass in the middle ear, mainly in the epitympanum with thinning and dehiscence of the tegmen tympani, assumed to be secondary to inflammatory process (Fig. 2).
Figure 2.
Coronal computed tomography scan of the left temporal bone showing the tegmen tympani defect.
According to the above history and findings, a preliminary diagnosis of chronic suppurative otitis media was made and a decision was taken to proceed with an exploratory mastoidectomy, via a wide postaural incision. During the procedure the osteomata of the external auditory canal were removed and the tympanic membrane was found to be intact with no signs of middle ear inflammation. However, a mass protruding through a dehiscence in the tegmen tympani was observed during mastoidectomy. This was excised flush to the tegmen dehiscence and the dura was elevated circumferentially. A temporalis fascia graft was used to seal the opening and a temporalis muscle inferiorly based pedicled flap was placed, in order to reinforce the reconstruction. Reconstruction of the osseous defect was not performed. Histology of the specimen confirmed an encephalocele. Follow-up at 12 months revealed no evidence of recurrence.
DISCUSSION
Etiological classification of skull base defects—and the resulting MECs—appears to be quite effective and simple (Table 1), although in some cases etiology may remain obscure. Various authors use the terms “congenital” and “spontaneous” quite loosely, assuming that all spontaneous MECs are formed through congenital dehiscences. However, it has not been defined whether these dehiscences are congenital or formed by bone remodeling or reabsorption during the patient's life. In this context, we find it more appropriate to divide spontaneous MECs into congenital (childhood onset) and idiopathic (adult onset).
Characterizing an MEC as post-traumatic or postinfective is quite straightforward. Conversely, in the absence of an appropriate medical history such as infection or trauma, an MEC could be safely categorized as spontaneous.10 However, it should be noted that occasionally head traumas may not be recorded or remembered. Temporal bone studies suggest that defects of the temporal bone are not uncommon. Kapur and Bangash11 performed a study of 50 temporal bones in a series of 25 pathology specimens with no history of chronic otitis media, cholesteatoma, or surgery. They reported 34% had skull base defects, of which 52.9% were located at the tegmen tympani and 5.8% at the tegmen mastoideum. Additionally no MECs were observed. Ahren and Thulin12 present similar results on autopsy specimens, with perforations of the tegmen tympani in 21% of cases. This discrepancy of incidence between bony skull base dehiscences and MECs indicates that other factors—apart from the existence of a skull base defect—participate in the pathogenesis and formation of MECs.
Normal dura is structurally capable of supporting brain, even over large defects, as observed in tegmen and posterior fossa dehiscences in revision mastoid surgery.13,14,15 Apparently, loss of structural dural integrity participates in the formation of MECs. The exact mechanism of MEC formation is still elusive; however, it is assumed that in areas of pre-existing bony defects, the chronic effect of normal intracranial pressure and pulsations cause gradual thinning and eventually loss of dural integrity16,17 with resulting cerebrospinal fluid (CSF) leaks and MECs. Possibly this same mechanism may lead to the creation of new dehiscences or the enlargement of pre-existing ones. It is believed that aberrant arachnoid villi of the middle and posterior surface dura may participate in the loss of dural integrity.18,19,20
The diagnosis of spontaneous temporal bone MECs may be quite challenging. Symptoms may include conductive hearing loss, otorrhea, and recurrent meningitis.13 Other less common presenting symptoms are facial nerve weakness, seizures,21,22 and even pulsatile tinnitus.23 Conductive hearing loss may be due to CSF or herniated cranial contents filling the middle ear cavity. Up to 25% of adult-onset CSF leaks present with meningitis.24 Therefore, in nonimmunocompromised patients with episodes of recurrent meningitis, where upper airway bacteria are isolated, with no apparent causative factor, the possibility of an occult MEC should be considered.13 It is quite common for these patients to be misdiagnosed as suffering from chronic otitis media or even otitis media with effusion. These patients receive the full range of medical treatment for otitis media; occasionally even ventilation tubes, from which a copious clear fluid is produced.1,22,25 Constant discharge of a clear watery fluid refractory to treatment is suggestive of a CSF leak. A beta-2-transferrin assay is quite effective in defining the true nature of the discharge.26 Our patient reported all the typical presenting symptoms: intermittent, clear, watery otorrhea, deafness, and episodes of meningitis. However, diagnosis remained elusive, which as mentioned above is not uncommon. Therefore a high index of suspicion and clinical impression should lead to the appropriate diagnosis.
Definite diagnosis, as well as preoperative planning, require appropriate radiological work-up. High-resolution computed tomography (HR-CT) can delineate quite efficiently anatomic relationships of the middle ear, as well as bone destruction. In retrospect, in the presented case absence of either a tympanic membrane perforation or ossicular chain erosion, as well as the presence of an intact scutum, made the diagnosis of chronic otitis media and cholesteatoma less likely.27,28,29 However, the nature of a soft tissue density mass, be it cholesteatoma, CSF, MEC, or granulation tissue, cannot be defined by CT, necessitating an MRI scan.14,25 Additionally dehiscences of the thin tegmen may not be apparent with an axial CT, as the tegmen is parallel to the axial projection, making coronal planes necessary as well.14 On MRI scan, dural herniation is indicated by the presence of a low-signal mass protruding via the dehiscence, bordered by high-signal CSF on T2-weighted images. On gadolinium-enhanced T1 spin-echo scans, brain tissue is herniating via the defect and the surrounding meninges are enhanced.30 According to Kaseff and associates,14 the diagnostic radiological approach in cases of suspected CSF leak or MEC should be done stepwise; initially an axial and coronal HR-CT of the temporal bone is performed in order to locate any possible tegmen tympani defects. If no dehiscences are found, no further investigation is required. However, in the presence of erosion, further sagittal or coronal MRI scan is required, with 3-mm sections, HR factors, and T1-weighted techniques.
Once the diagnosis of an MEC has been made, prompt surgical repair is indicated. Sdano and Pensak25 have summarized the goals of surgical treatment:
Management of the herniated cranial contents
Occlusion of the bony defect, with a seal that will withstand intracranial pressure (< 200 mm H2O), to prevent recurrence
Restoration of the conductive hearing mechanism, if possible
The herniated brain material due to longstanding ischemia is devitalized and considered functionless.11,31,32,33,34,35 Therefore reduction of the material back into the cranial cavity is not warranted, and the majority of authors suggest amputation.15,25,36,37 Removal of the herniated tissue is considered to be safe, as the majority of authors report no postoperative consequences. However, some reports of complications may be found in the literature.1,32 It should also be noted that some authors support the opinion that small viable hernias in a noninfected environment could be replaced intracranially.1,22,30,32,38
Temporal bone MECs can be approached either through the mastoid, through the middle cranial fossa, or through both.1,36,38,39 Factors to be considered in choosing an approach include defect size and volume of herniated brain. Small MECs can be repaired by a mastoidectomy approach and the bony defect closed in a layered fashon.39,40 The use of autologous biologic material for the repair is suggested.41 Various techniques are described, such as the use of fascia sewn to dura,42,43 rotational temporalis muscle flap,35 temporalis fascia and split thickness bony skin grafts.44 The skull base defect can be sealed by bone grafts or cartilage. However, due to limited exposure, larger MECs and bony dehiscences are approached either from the middle fossa or using a combined approach. In the presented case we used a transmastoid approach. The defect site was sealed by a combination of temporalis fascia and muscle. As the tympanic membrane and the ossicles were intact no restoration of the hearing mechanism was deemed necessary. The mastoid cavity was not obliterated.
As mentioned earlier, the management of MECs is surgical. Considering the high incidence of meningitis in patients with MECs, as well as the high morbidity and mortality of meningitis, a question arises as to whether prophylactic antibiotics should be administered. This subject remains controversial, as such therapeutic regimes have not led to a significant reduction of meningitis,45 although there are series of reported decrease following antibiotic prophylaxis.46 Additionally, the disturbance of the rhinopharyngeal flora possibly could trigger further infections and increased incidence of gram-negative bacterial infections.47,48
CONCLUSION
Idiopathic MECs, however rare, may present during adulthood with symptoms such as clear-fluid ear discharge, meningitis, or middle ear soft-tissue masses. Therefore the otorhinolaryngologist should be familiar with this condition, in order to facilitate prompt diagnosis and treatment. CT and MRI scans are effective diagnostic tools and obligatory for presurgical planning. The concept of surgical repair is to obtain appropriate exposure via either the mastoid or the middle cranial fossa, or both, and to provide a strong layered seal.
REFERENCES
- Jackson C G, Pappas D G, Jr, Manolidis S, et al. Brain herniation into the middle ear and mastoid: concepts in diagnosis and surgical management. Am J Otol. 1997;18:198–205. discussion 205–206. [PubMed] [Google Scholar]
- MacRae D L, Ruby R F. Recurrent meningitis secondary to perilymph fistula in young children. J Otolaryngol. 1990;19:222–225. [PubMed] [Google Scholar]
- Phillipps J J. Bilateral oval window fistulae with recurrent meningitis. J Laryngol Otol. 1986;100:329–331. doi: 10.1017/s0022215100099229. [DOI] [PubMed] [Google Scholar]
- Quiney R E, Mitchell D B, Djazeri B, Evans J G. Recurrent meningitis in children due to inner ear abnormalities. J Laryngol Otol. 1989;103:473–480. doi: 10.1017/s002221510015666x. [DOI] [PubMed] [Google Scholar]
- Barr B, Wersall J. Cerebrospinal otorrhea with meningitis in congenital deafness. Arch Otolaryngol. 1965;81:26–28. doi: 10.1001/archotol.1965.00750050031008. [DOI] [PubMed] [Google Scholar]
- Bennett R J. On subarachnoid-tympanic fistulae: a report of two cases of the rare indirect type. J Laryngol Otol. 1966;80:1242–1252. doi: 10.1017/s0022215100066627. [DOI] [PubMed] [Google Scholar]
- Biggers W P, Howell N N, Fisher N D, Himadi G M. Congenital ear anomalies associated with otic meningitis. Arch Otolaryngol. 1973;97:399–401. doi: 10.1001/archotol.1973.00780010411010. [DOI] [PubMed] [Google Scholar]
- Tschiang H H, Harrison M S, Ozsahinaglu C A. Cerebro-spinal otorrhoea. J Laryngol Otol. 1973;87:475–483. doi: 10.1017/s002221510007715x. [DOI] [PubMed] [Google Scholar]
- Weider D J, Geurkink N A, Saunders R L. Spontaneous cerebrospinal fluid otorhinorrhea. Am J Otol. 1985;6:416–422. [PubMed] [Google Scholar]
- Schick B, Draf W, Kahle G, Weber R, Wallenfang T. Occult malformations of the skull base. Arch Otolaryngol Head Neck Surg. 1997;123:77–80. doi: 10.1001/archotol.1997.01900010087013. [DOI] [PubMed] [Google Scholar]
- Kapur T R, Bangash W. Tegmental and petromastoid defects in the temporal bone. J Laryngol Otol. 1986;100:1129–1132. doi: 10.1017/s0022215100100702. [DOI] [PubMed] [Google Scholar]
- Ahren C, Thulin C A. Fatal intracranial complications due to politzerization of the outer ear canal in otitis therapy, caused by intracranial temporal bone defects. Sven Lakartidn. 1964;61:2421–2437. [PubMed] [Google Scholar]
- Kamerer D B, Caparosa R J. Temporal bone encephalocele: diagnosis and treatment. Laryngoscope. 1982;92:878–882. [PubMed] [Google Scholar]
- Kaseff L G, Seidenwurm D J, Nieberding P H, Nissen A J, Remley K R, Dillon W. Magnetic resonance imaging of brain herniation into the middle ear. Am J Otol. 1992;13:74–77. [PubMed] [Google Scholar]
- Mosnier I, Fiky L E, Shahidi A, Sterkers O. Brain herniation and chronic otitis media: diagnosis and surgical management. Clin Otolaryngol Allied Sci. 2000;25:385–391. doi: 10.1046/j.1365-2273.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- Kaufman B, Yonas H, White R J, Miller C F. Acquired middle cranial fossa fistulas: normal pressure and nontraumatic in origin. Neurosurgery. 1979;5:466–472. doi: 10.1227/00006123-197910000-00011. [DOI] [PubMed] [Google Scholar]
- Ommaya A K. Cerebrospinal fluid rhinorrhea. Neurology. 1964;14:106–113. doi: 10.1212/wnl.14.2.106. [DOI] [PubMed] [Google Scholar]
- Sckunecht H F, Gulya A J. Anatomy of the Temporal Bone with Surgical Implication. Philadelphia, PA: Lea & Febiger; 1986. pp. 125–126.
- Gacek R R. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1990;99:854–862. doi: 10.1177/000348949009901102. [DOI] [PubMed] [Google Scholar]
- Gacek R R. Evaluation and management of temporal bone arachnoid granulations. Arch Otolaryngol Head Neck Surg. 1992;118:327–332. doi: 10.1001/archotol.1992.01880030119024. [DOI] [PubMed] [Google Scholar]
- Lalwani A K. In: Jackler RK, Brackman DE, editor. Neurotology. St. Louis, MO: Mosby; 2004. Temporal bone encephalocele. pp. 1089–1095.
- Lundy L B, Graham M D, Kartush J M, LaRouere M J. Temporal bone encephalocele and cerebrospinal fluid leaks. Am J Otol. 1996;17:461–469. [PubMed] [Google Scholar]
- Kale S U, Pfleiderer A G, Cradwick J C. Bilateral defects of the tegmen tympani associated with brain and dural prolapse in a patient with pulsatile tinnitus. J Laryngol Otol. 2000;114:861–863. doi: 10.1258/0022215001904176. [DOI] [PubMed] [Google Scholar]
- Pappas D G, Jr, Hoffman R A, Cohen N L, Pappas D G., Sr Spontaneous temporal bone cerebrospinal fluid leak. Am J Otol. 1992;13:534–539. [PubMed] [Google Scholar]
- Sdano M T, Pensak M L. Temporal bone encephaloceles. Curr Opin Otolaryngol Head Neck Surg. 2005;13:287–289. doi: 10.1097/01.moo.0000179247.51476.f5. [DOI] [PubMed] [Google Scholar]
- Skedros D G, Cass S P, Hirsch B E, Kelly R H. Beta-2 transferrin assay in clinical management of cerebral spinal fluid and perilymphatic fluid leaks. J Otolaryngol. 1993;22:341–344. [PubMed] [Google Scholar]
- Mafee M F, Kumar A, Yannias D A, Valvassori G E, Applebaum E L. Computed tomography of the middle ear in the evaluation of cholesteatomas and other soft tissue masses: comparison with pluridirectional tomography. Radiology. 1983;148:465–472. doi: 10.1148/radiology.148.2.6867344. [DOI] [PubMed] [Google Scholar]
- Ishii K, Takahashi S, Matsumoto K, et al. Middle ear cholesteatoma extending into the petrous apex: evaluation by CT and MR imaging. AJNR Am J Neuroradiol. 1991;12:719–724. [PMC free article] [PubMed] [Google Scholar]
- Liu D P, Bergeron R T. Contemporary radiologic imaging in the evaluation of middle ear-attic-antral complex cholesteatomas. Otolaryngol Clin North Am. 1989;22:897–909. [PubMed] [Google Scholar]
- Raine C. Diagnosis and management of otologic cerebrospinal fluid leak. Otolaryngol Clin North Am. 2005;38:583–595. doi: 10.1016/j.otc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Graham M D, Lundy L B. In: Brackmann DE, Shelton C, Arriaga M, editor. Otologic Surgery. Philadelphia, PA: WB Saunders; 1993. Dural herniation and cerebrospinal fluid leaks. pp. 277–288.
- Glasscock M E, III, Dickins J R, Jackson C G, Wiet R J, Feenstra L. Surgical management of brain tissue herniation into the middle ear and mastoid. Laryngoscope. 1979;89:1743–1754. doi: 10.1288/00005537-197911000-00005. [DOI] [PubMed] [Google Scholar]
- Jahrsdoerfer R A, Richtsmeier W J, Cantrell R W. Spontaneous CSF otorrhea. Arch Otolaryngol. 1981;107:257–261. doi: 10.1001/archotol.1981.00790400059015. [DOI] [PubMed] [Google Scholar]
- Ramsden R T, Latif A, Lye R H, Dutton J E. Endaural cerebral hernia. J Laryngol Otol. 1985;99:643–651. doi: 10.1017/s0022215100097413. [DOI] [PubMed] [Google Scholar]
- Dedo G G, Sooy F A. Endaural encephalocele and cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1970;79:168–177. doi: 10.1177/000348947007900116. [DOI] [PubMed] [Google Scholar]
- Lalwani A K. In: Jackler RK, Brackman DE, editor. Neurotology. St. Louis, MO: Mosby; 2004. Temporal bone encephalocele. pp. 1089–1095.
- Neely J G, Neblett C R, Rose J. Diagnosis and treatment of spontaneous cerebrospinal fluid otorrhea. Laryngoscope. 1982;92:609–612. doi: 10.1002/lary.1982.92.6.609. [DOI] [PubMed] [Google Scholar]
- Wootten C T, Kaylie D M, Warren F M, Jackson C G. Management of brain herniation and cerebrospinal fluid leak in revision chronic ear surgery. Laryngoscope. 2005;115:1256–1261. doi: 10.1097/01.MLG.0000165455.20118.E3. [DOI] [PubMed] [Google Scholar]
- Kuhweide R, Casselman J W. Spontaneous cerebrospinal fluid otorrhea from a tegmen defect: transmastoid repair with minicraniotomy. Ann Otol Rhinol Laryngol. 1999;108:653–658. doi: 10.1177/000348949910800706. [DOI] [PubMed] [Google Scholar]
- Valtonen H, Geyer C, Tarlov E, Heilman C, Poe D. Tegmental defects and cerebrospinal fluid otorrhea. ORL J Otorhinolaryngol Relat Spec. 2001;63:46–52. doi: 10.1159/000055705. [DOI] [PubMed] [Google Scholar]
- Savva A, Taylor M J, Beatty C W. Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope. 2003;113:50–56. doi: 10.1097/00005537-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Dandy W E. Treatment of rhinorrhea and otorrhea. Arch Surg. 1944;49:779–788. [Google Scholar]
- Canfeield R B. Some conditions associated with the loss of cerebrospinal fluid. Ann Otol Rhinol Laryngol. 1913;22:604–622. [Google Scholar]
- Paparella M M, Meyerhoff W L, Oliviera C A. Mastoiditis and brain hernia (mastoiditis cerebri) Laryngoscope. 1978;88:1097–1106. doi: 10.1002/lary.1978.88.7.1097. [DOI] [PubMed] [Google Scholar]
- Rathore M H. Do prophylactic antibiotics prevent meningitis after basilar skull fracture? Pediatr Infect Dis J. 1991;10:87–88. doi: 10.1097/00006454-199102000-00001. [DOI] [PubMed] [Google Scholar]
- Friedman J A, Ebersold M, Quast L M. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062–1066. doi: 10.1007/s00268-001-0059-7. [DOI] [PubMed] [Google Scholar]
- Ignelzi R J, VanderArk G D. Analysis of the treatment of basilar skull fractures with and without antibiotics. J Neurosurg. 1975;43:721–726. doi: 10.3171/jns.1975.43.6.0721. [DOI] [PubMed] [Google Scholar]
- Eljamel M S. Antibiotic prophylaxis in unrepaired CSF fistulae. Br J Neurosurg. 1993;7:501–505. doi: 10.3109/02688699308995072. [DOI] [PubMed] [Google Scholar]