ABSTRACT
The management of clival chordoma remains problematic. We present the case of a 48-year-old woman with clival chordoma who underwent multiple surgeries and radiation therapy, including gamma knife stereotactic radiosurgery (GK-SRS), during a 10-year clinical course. The tumor was initially removed by gross total resection via the trans-sphenoidal approach, followed by external linac radiation therapy. The tumor recurred at the clivus 5 years after the initial operation. After repeated trans-sphenoidal removal of recurrent tumors, she twice underwent GK-SRS for a tumor remnant adjacent to the brainstem. Although this part of the tumor was controlled by GK-SRS, there was further tumor extension toward the sphenoid and maxillary sinuses. Ultimately, lower cranial nerve dysfunction developed due to tumor extension into the lower part of the clivus and the patient died of respiratory failure. Autopsy revealed the tumor to extend from the lower clivus to the bilateral middle fossae. The lower part of the tumor extended to the nasal cavity and to the posterior wall of the pharynx, resulting in compression of the upper pharyngeal region. The tumor around the jugular foramen compressed the lower cranial nerves bilaterally. Tumor cells did not, however, invade the intradural space microscopically. Although chordoma is not biologically malignant, this tumor can show massive extension with destruction of bony structures and extracranial invasion of connective tissues. Therefore, the optimal treatment strategy is to remove the tumor mass as extensively as possible, including normal bony structures and connective tissues surrounding the tumor, using skull base surgical techniques.
Keywords: Chordoma, autopsy, skull base surgery, gamma knife radiosurgery, proton radiation therapy
Chordoma, a rare tumor arising from the remnant of the embryonic notochord along the spinal axis, accounts for 0.2 to 0.8% of all intracranial tumors.1 Although this tumor shows slow growth biologically, chordomas are well known to destroy bony structures and invade surrounding tissue.1,2,3,4,5 Therefore, developing surgical techniques and other strategies for tumor removal is absolutely crucial for mass reduction, and adjuvant therapies such as various forms of radiation are also essential for tumor control.1,6,7,8,9,10,11
We present a female patient with clival chordoma who underwent multiple surgeries and radiation during a 10-year clinical course. We review the clinical features and macroscopic and microscopic pathological findings at autopsy of our case and discuss the treatment strategies based on these pathological findings.
CASE REPORT
Clinical Course
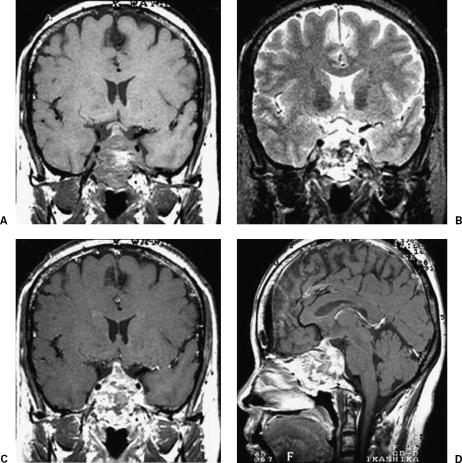
A 48-year-old woman presented with left visual disturbance and diplopia, both due to left oculomotor and abducence nerve palsy, which had developed in October of 1994. Magnetic resonance (MR) imaging revealed an enhanced mass, with low-signal intensity on T1-weighted images and high-signal intensity on T2-weighted images which extended from the sphenoid sinus to the clivus (Fig. 1). Intratumoral hemorrhage was also seen as high-signal intensity on T1-weighted and low-signal intensity on T2-weighted images. Histopathological examination by intranasal biopsy revealed a chordoma. The tumor was removed by gross total resection, via the trans-sphenoidal approach, on January 20, 1995. Left visual acuity and diplopia improved postoperatively. Linac external local radiation therapy, with a total dose of 55.2 Gy, was administered postoperatively. The patient subsequently did well for 5 years, but follow-up MR imaging in January of 2000 revealed tumor recurrence at the clivus with tumor extension to the upper pharyngeal space. Recurrent tumors were twice removed, in March and December of 2000, via the trans-sphenoidal approach (Table 1). The patient underwent gamma knife radiosurgery (GK-SRS) with a marginal dose of 15.0 Gy (60% isodose gradient) for a tumor remnant located close to the brainstem on January 13, 2001 (Fig. 2) (Table 1). At that time, the tumor extended to the bilateral maxillary sinuses, with persistent epistaxis necessitating surgical removal via a LeFort I osteotomy in October of 2001. A second GK-SRS targeting a tumor protrusion toward the brainstem was performed, with a 15.0 Gy marginal dose (60% isodose gradient), on September 14, 2002 (Fig. 2). However, right visual acuity impairment due to tumor extension into the right orbit as well as the right maxillary and posterior ethmoid sinuses was demonstrated in February of 2003. This tumor regrowth was partially removed via a combined craniofacial approach. Visual disturbance improved postoperatively. However, swelling of the soft palate and lower cranial nerve dysfunction appeared in August 2003. MR imaging showed continuous tumor growth with extension to the lower clival region and intramaxillary sinuses bilaterally (Fig. 3). The patient died of respiratory failure on May 9, 2004.
Figure 1.
Magnetic resonance images of the clival tumor before the initial operation. (A) T1-weighted, (B) T2-weighted, and (C,D) T1-weighted images with contrast medium. The tumor shows high-signal intensity on the T1-weighted image and low-signal intensity on the T2-weighted image, probably due to intratumoral hemorrhage. The tumor was enhanced with gadolinium-DTPA.
Table 1.
History of Tumor Extension and Therapy
| Treatment Date | Tumor Extension | Clinical Symptoms | Treatment (Operative Approach) |
|---|---|---|---|
| January 1995 | Sphenoid sinus, Clivus | Left visual disturbance | Trans-sphenoidal |
| Palsy of left nerves III and VI | |||
| March 2000 | Clivus, Nasal cavity | Palsy of left nerve VI | Trans-sphenoidal |
| December 2000 | Sphenoid sinus | Trans-sphenoidal | |
| January 2001 | Protrusion toward brainstem | Gamma knife radiosurgery | |
| October 2001 | Maxillary sinuses (bilateral) | Epistaxis | LeFort I osteotomy |
| September 2002 | Protrusion toward brainstem | Gamma knife radiosurgery | |
| February 2003 | Right orbital cavity, Nasal cavity | Right visual disturbance | Transcranial and transfacial |
| August 2003 | Lower clivus, Maxillary sinus-Laryngeal space | Lower cranial nerve dysfunction | |
| Laryngeal space | |||
| May 2004 | Death (autopsy) |
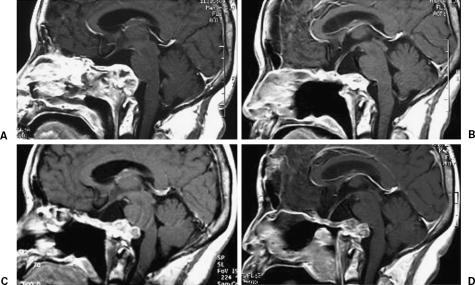
Figure 2.
Effects of gamma knife radiosurgery for the recurrent tumor at the clivus. (A) Image was obtained before the initial gamma knife radiosurgery and (B) 12 months after radiosurgery. (C) The tumor again showed regrowth at 19 months after the initial radiosurgery. (D) Image was obtained 13 months after the second radiosurgery.
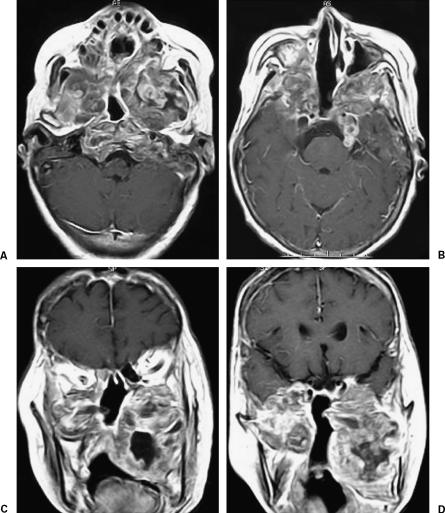
Figure 3.
(A–D) Magnetic resonance images obtained shortly before the patient's death. T1-weighted magnetic resonance images with contrast medium reveal massive tumor growth extracranially. The tumor has extended to the bilateral maxillary sinuses, and thereby compresses the nasal and oral cavities and the lower part of the clivus, resulting in lower cranial nerve dysfunction.
Histopathological Findings at Surgery and at Autopsy
Tumor specimens obtained during the first operation showed cellular lobules made up of mucin-containing cells microscopically. Multivacuolated or physaliphorous cells were also seen. Myxoid matrix was positive for Alcian blue staining. These findings were compatible with the diagnosis of chordoma. Specimens from repeated surgeries for recurrent tumors had an appearance similar to those obtained during the first operation. MIB-1 staining indices were less than 5% and did not increase with tumor recurrence or invasion of surrounding tissues.
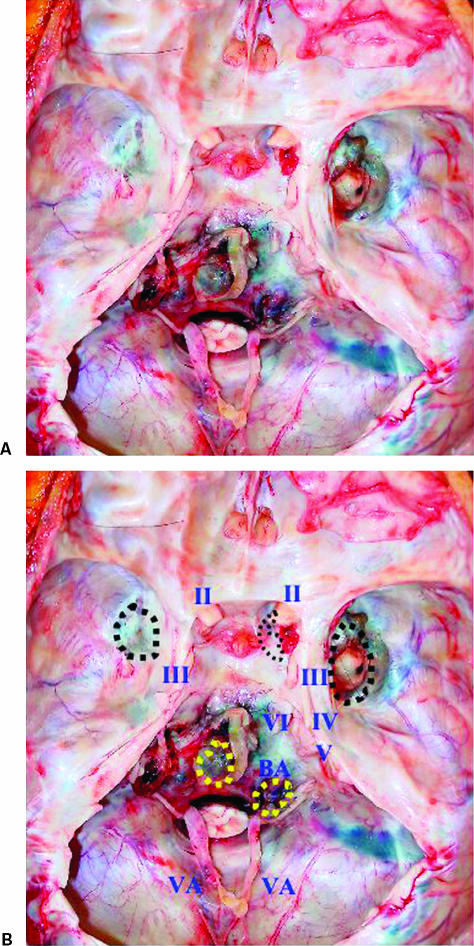
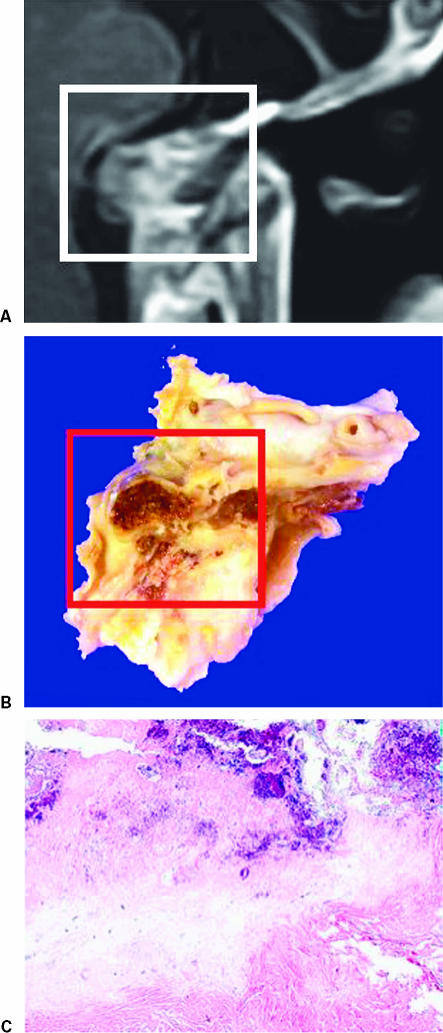
At autopsy, the tumor was macroscopically located at four extracranial skull base sites; upper clivus, lower clivus, and right and left middle fossae (Fig. 4). The tumor at the mid clivus was close to the left sixth, seventh, and eighth cranial nerves and the brainstem. However, the dura mater of the skull base was intact. The tumor at the lower clivus compressed the ninth, tenth, eleventh, and twelfth cranial nerves bilaterally. The tumor at the right middle fossa was close to the cavernous sinus, the lateral wall of the optic canal, and the posterior wall of the orbital cavity. The tumor at the left middle fossa was located extradurally near the foramen ovale. All skull base tumors were located extradurally and the dura mater itself was intact macroscopically (Fig. 4). Bony structures at the pituitary fossa, clivus, and sphenoid sinus showed thinning. Both the dorsum sellae and the posterior clinoid process had disappeared and the pituitary gland was not visible, probably due to severe atrophy (Fig. 5). The dura mater in the pituitary fossa was also intact macroscopically. Additional extracranial tumors were also seen in the sphenoid, as well as the bilateral ethmoid and maxillary sinuses, and extended to the nasal cavity with compression of the posterior wall of the pharynx resulting in stenosis of the upper pharyngeal space.
Figure 4.
(A,B) Photographs obtained at autopsy showing the internal skull base. The dura mater is uniformly intact and tumor tissue adheres to four locations extradurally (dotted circle): the bilateral middle fossae and the mid and lower portions of the clivus. The pituitary fossa and the posterior clinoid process show thinning and the pituitary gland cannot be visualized. The tumor around the lower clivus compresses the lower cranial nerves bilaterally.
Figure 5.
(A) Final magnetic resonance image, (B) macroscopic view of the tumor at the thinned portion of the clivus at autopsy, and (C) microscopic photograph of the lesion at the center of the clivus. Microscopically, the majority of this lesion at the clivus was composed of necrotic tissue with only a small area of viable tumor cells. The dura mater of the pituitary fossa was intact.
Histopathological findings obtained at autopsy were essentially consistent with the operative samples. Mucin-containing cells, multivacuolated or physaliphorous cells were seen. The dura mater of the pituitary fossa was intact and appeared to form a barrier against tumor invasion (Fig. 5). Therefore, dissemination of tumor cells into the subarachnoid space was apparently negative. The MIB-1 labeling index was under 1% with no evidence of malignancy.
Effects of Gamma Knife Radiosurgery
The patient underwent GK-SRS twice for treatment of remnant or recurrent tumors (Table 1). For both treatment sessions, the tumor was covered with a 60% isodose gradient and the dose delivered to the tumor margin was 15 Gy. The dose delivered to the adjacent brainstem was kept below 12.5 Gy under both conditions. After the first GK-SRS, no neurological deterioration was seen. MR imaging showed a decreased tumor volume (Fig. 2B). Nineteen months later, however, the tumor again showed regrowth (Fig. 2C). The patient underwent a second GK-SRS with the same protocol. Despite lack of tumor shrinkage (Fig. 2D), no additional neurological deficits were seen after this second treatment. The adjacent brain stem showed no MR imaging evidence of adverse effects of radiosurgery after either treatment.
DISCUSSION
The patient described herein survived for a relatively long time. However, multiple surgical interventions and two stereotactic radiosurgeries were necessitated by recurrence 5 years after the initial operation (Table 1). With recurrence, the tumor continued to grow and progressively invaded surrounding structures including the paranasal sinuses, nasal cavities, orbital cavity, and pharyngeal space during this patient's 10-year clinical course. Cranial nerve functions were also impaired due to extradural tumor compression. The patient ultimately died of respiratory failure caused by lower cranial nerve dysfunction.
Although chordomas are basically extra-axial, biologically slow-growing, and nonmalignant histopathologically, management of these tumors is still problematic because they grow close to critical structures, making surgical approaches complex and difficult. Moreover, they often recur even after gross total removal at the initial operation. Thus, the behavior of chordomas is referred to as “clinically malignant.”
Several publications have focused on the control of these lesions, and have described surgical removal using a variety of techniques12,13,14,15 and postoperative adjuvant therapies, including various types of radiation therapy.1,6,7,8,9,10,11 Historically, these tumors have been associated with a grim, or even hopeless, prognosis. Falconer et al advocated a classification of chordomas in 1968, but their surgical results were not satisfactory.3 Krayenbuhl and Yasargil reported a mean survival of 3 years in 1975.16 Since the advent of imaging technologies, such as computed tomography and MR imaging, and the introduction of advanced operative techniques including microsurgery and skull base surgery, the extent of surgical resection and patient prognosis have been improving.8,17,18 Gay and associates emphasized the importance of surgical removal and reported that patients with total or near-total resection had a lower risk of recurrence and a longer survival time than patients undergoing subtotal or partial resection.17
Chordomas generally do not invade the dura mater or the intradural space, according to reported autopsy findings.2,19 Although these tumors usually destroy bony structures of the skull base, such as the sella turcica and clivus, the dura mater is uniformly intact. This was also the case in our patient, in whom the skull base had thinned but the dura mater was intact and there was no invasion of the dura mater by tumor cells. Oikawa and colleagues also found that the tumor invaded the submucous layer without invading the dura itself or vital neurovascular structures, even in the advanced stage.2 The dura mater is certainly an effective barrier against tumor invasion of the intradural space. Based on both surgical results described in previous reports and pathological information obtained from autopsy cases, maximum resection of the primary tumor with not only bone and submucous structures showing tumor invasion but also normal bone and soft tissue surrounding the tumor, as extensively as possible, is considered to be crucial for optimal initial surgical treatment.
Although chordomas have been considered to be radioresistant,4,5,16 postoperative radiation therapy has been shown to have a beneficial effect.1,8,9 In particular, when there is residual tumor tissue after the initial operation, various types of radiation therapy thought to play important roles in the first-line management strategy include stereotactic fractionated radiotherapy,10 GK-SRS,7,20,21 and proton and photon radiotherapy.18,22,23,24 Our patient underwent 55.2 Gy of conventional external radiotherapy postoperatively followed by a disease-free interval of 5 years. This would appear to be a very satisfactory result. Zorlu and coworkers reported the effect of conventional external radiotherapy in the management of 18 clivus chordomas with overt residual disease, and concluded incomplete surgery and conventional external radiotherapy at a dose of ~60 Gy to apparently be inadequate.6 The difference between our case and those reported by Zorlu et al may reflect the importance of gross total tumor resection and the limitations of external conventional radiation therapy. Stereotactic radiosurgery combines the accuracy of stereotactic guidance with the advantages of high-dose single-session irradiation. Kondziolka and associates evaluated the effectiveness of this radiation system for chordomas, using GK-SRS, and reported radiosurgery to be a safe and effective treatment when the target volume is small.7 Maximal tumor resection followed by early GK-SRS for the small residual tumor volume has since been advocated as the first-line management for chordomas.20,21,25 Pamir and colleagues also used GK-SRS for 7 patients among 19 survivors with 26 skull base chordomas. The tumor control rate was 71.4% (1 decrease, 4 no change, 2 increase in tumor volume) with a 23.3-month mean follow-up.20 Feigl et al also reported a local tumor control rate of 93.3% in their series of 3 chordoma and 10 chondrosarcoma patients.21 In both reports, however, a longer follow-up would appear to be necessary to confirm the efficacy of this treatment approach for chordomas.
On the other hand, Suit et al advocated the use of a postoperative proton radiation system for chordomas.11 They reported that local control was achieved for all 10 of their patients, 6 with chordoma, 3 with chondrosarcoma and 1 with a neurofibrosarcoma, with follow-up periods ranging from 2 months to 6 years. Austin-Seymour et al also reported the 5-year actuarial local control rate to be 82% and the disease-free survival rate to be 76% in 68 patients with chordoma or low-grade chondrosarcoma followed for at least 17 months and for a median of 34 months.26 Based on these initial results, several investigators began to examine the effectiveness of proton radiation therapy for chordomas.22,24,26,27 Hug et al obtained excellent results, with a local control rate of 76% for 33 chordomas and a 5-year survival rate of 79% with postoperative proton radiation therapy24. Colli and Al-Mefti also obtained optimal results using adjuvant proton-beam therapy after extensive resection and treatments for chordomas.22 Using this radiation system, the 5-year survival rate for chordoma patients reached 72.2% to 85.9%.22,24,26,27 Certain complications, such as radiation-induced necrosis, osteonecrosis of the temporal bone, and damage to the visual apparatus and pituitary function,22,26 have been reported but proton radiation therapy presently appears to be the best choice as a postoperative adjuvant therapy in the first-line management of chordomas.
A far more serious problem in the management of chordomas is the best treatment strategy for recurrent lesions. As in our case, once a tumor recurs, surgical removal tends to be palliative, aimed only at relieving neurological symptoms, and the intervals between operations become progressively shorter (Table 1). Few reports have focused on the treatment of recurrent lesions. Feigl and associates advocated a multimodal combination of surgery and early postoperative GK-SRS as the first-line treatment and also recommended this strategy for tumor recurrences.21 Two GK-SRS sessions for a tumor remnant or regrowth adjacent to the brainstem were useful in our case and neither treatment was associated with adverse effects. Histopathological examination of the autopsy specimen revealed the lesion at the clivus to be mostly necrotic tissue with only a small portion of the tumor cells being viable. The reason for the treatment failure in our case was continuous tumor growth outside the GK-SRS treated area. Taking all of the observations made in the present case together, maximum tumor resection at the initial operation followed by proton radiation therapy should be performed as the first-line treatment for chordomas and GK-SRS for small recurrent tumors.
CONCLUSION
In conclusion, management of chordomas remains a challenge even at the present time. However, we must continue to deal aggressively with these tumors, using advanced surgical techniques and treatment modalities such as postoperative proton-beam radiation therapy and GK-SRS.
ACKNOWLEDGMENTS
We reported this case at the 17th Annual Meeting of the Japanese Society for Skull Base Surgery on July 7–8, 2005. The authors thank Biert E. Barfud, M.D., Katsuta Hospital Mito Gamma House, for her assistance in the preparation of this article.
REFERENCES
- Heffelfinger M J, Dahlin D C, MacCarty C S, Beabout J W. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973;32:410–420. doi: 10.1002/1097-0142(197308)32:2<410::aid-cncr2820320219>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Kyoshima K, Goto T, et al. Histological study on local invasiveness of clival chordoma: case report of autopsy. Acta Neurochir (Wien) 2001;143:1065–1069. doi: 10.1007/s007010170013. [DOI] [PubMed] [Google Scholar]
- Falconer M A, Bailey I C, Duchen L W. Surgical treatment of chordoma and chondroma of the skull base. J Neurosurg. 1968;29:261–275. doi: 10.3171/jns.1968.29.3.0261. [DOI] [PubMed] [Google Scholar]
- Rich T A, Schiller A, Suit H D, Mankin H J. Clinical and pathologic review of 48 cases of chordoma. Cancer. 1985;56:182–187. doi: 10.1002/1097-0142(19850701)56:1<182::aid-cncr2820560131>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sen C N, Sekhar L N, Schramm V L, Janecka I P. Chordoma and chondrosarcoma of the cranial base: an 8-year experience. Neurosurgery. 1989;25:931–941. doi: 10.1097/00006123-198912000-00013. [DOI] [PubMed] [Google Scholar]
- Zorlu F, Gurkaynak M, Yildiz F, Oge K, Atahan I L. Conventional external radiotherapy in the management of clivus chordomas with overt residual disease. Neurol Sci. 2000;21:203–207. doi: 10.1007/s100720070077. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford L D, Flickinger J C. The role of radiosurgery in the management of chordoma and chondrosarcoma of the cranial base. Neurosurgery. 1991;29:38–46. doi: 10.1097/00006123-199107000-00007. [DOI] [PubMed] [Google Scholar]
- Forsyth P A, Cascino T L, Shaw E G, et al. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg. 1993;78:741–747. doi: 10.3171/jns.1993.78.5.0741. [DOI] [PubMed] [Google Scholar]
- Favre J, Deruaz J, Uske A, Tribolet N. Skull base chordomas: presentation of six cases and review of the literature. J Clin Neurosci. 1994;1:7–18. doi: 10.1016/0967-5868(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Debus J, Schulz-Ertner D, Schad L, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys. 2000;47:591–596. doi: 10.1016/s0360-3016(00)00464-8. [DOI] [PubMed] [Google Scholar]
- Suit H D, Goitein M, Munzenrider J, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56:377–385. doi: 10.3171/jns.1982.56.3.0377. [DOI] [PubMed] [Google Scholar]
- al-Mefty O, Anand V K. Zygomatic approach to skull-base lesions. J Neurosurg. 1990;73:668–673. doi: 10.3171/jns.1990.73.5.0668. [DOI] [PubMed] [Google Scholar]
- al-Mefty O, Borba L A, Aoki N, Angtuaco E, Pait T G. The transcondylar approach to extradural nonneoplastic lesions of the craniovertebral junction. J Neurosurg. 1996;84:1–6. doi: 10.3171/jns.1996.84.1.0001. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Nanda A, Sen C N, Snyderman C N, Janecka I P. The extended frontal approach to tumors of the anterior, middle, and posterior skull base. J Neurosurg. 1992;76:198–206. doi: 10.3171/jns.1992.76.2.0198. [DOI] [PubMed] [Google Scholar]
- Sen C N, Sekhar L N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197–204. doi: 10.1097/00006123-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Krayenbuhl H, Yasargil M G. Cranial chordomas. Progr Neurol Surg. 1975;6:380–434. [Google Scholar]
- Gay E, Sekhar L N, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery. 1995;36:887–897. doi: 10.1227/00006123-199505000-00001. [DOI] [PubMed] [Google Scholar]
- al-Mefty O, Borba L A. Skull base chordomas: a management challenge. J Neurosurg. 1997;86:182–189. doi: 10.3171/jns.1997.86.2.0182. [DOI] [PubMed] [Google Scholar]
- Kakuno Y, Yamada T, Hirano H, Mori H, Narabayashi I. Chordoma in the sella turcica. Neurol Med Chir (Tokyo) 2002;42:305–308. doi: 10.2176/nmc.42.305. [DOI] [PubMed] [Google Scholar]
- Pamir M N, Kilic T, Ture U, Ozek M M. Multimodality management of 26 skull-base chordomas with 4-year mean follow-up: experience at a single institution. Acta Neurochir (Wien) 2004;146:343–354. doi: 10.1007/s00701-004-0218-3. [DOI] [PubMed] [Google Scholar]
- Feigl G C, Bundschuh O, Gharabaghi A, et al. Evaluation of a new concept for the management of skull base chordomas and chondrosarcomas. J Neurosurg. 2005;102(suppl):165–170. doi: 10.3171/jns.2005.102.s_supplement.0165. [DOI] [PubMed] [Google Scholar]
- Colli B, Al-Mefty O. Chordomas of the craniocervical junction: follow-up review and prognostic factors. J Neurosurg. 2001;95:933–943. doi: 10.3171/jns.2001.95.6.0933. [DOI] [PubMed] [Google Scholar]
- Crockard H A, Steel T, Plowman N, et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg. 2001;95:175–183. doi: 10.3171/jns.2001.95.2.0175. [DOI] [PubMed] [Google Scholar]
- Hug E B, Loredo L N, Slater J D, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–439. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- Muthukumar N, Kondziolka D, Lunsford L D, Flickinger J C. Stereotactic radiosurgery for chordoma and chondrosarcoma: further experiences. Int J Radiat Oncol Biol Phys. 1998;41:387–392. doi: 10.1016/s0360-3016(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Austin-Seymour M, Munzenrider J, Linggood R, et al. Fractionated proton radiation therapy of cranial and intracranial tumors. Am J Clin Oncol. 1990;13:327–330. doi: 10.1097/00000421-199008000-00013. [DOI] [PubMed] [Google Scholar]
- Igaki H, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60:1120–1126. doi: 10.1016/j.ijrobp.2004.05.064. [DOI] [PubMed] [Google Scholar]