ABSTRACT
Objectives: This study sought to determine explicitly whether postsurgical facial nerve outcomes for patients with a cystic component to a vestibular schwannoma were significantly different from those with a solid tumor. Design: Seventy patients who underwent translabyrinthine surgery for a cystic vestibular schwannoma between May 1981 and the present, and who had complete records in our database, were identified. These were compared with a group of patients with solid tumors matched to the study group on the following parameters: House-Brackmann grade at presentation, tumor size, surgical approach, age. Setting: Regional tertiary referral center. Participants: Adult patients with vestibular schwannomas. Main Outcome Measures: House-Brackmann score 2 years following surgery. Results: No significant difference was found between the two groups. Conclusions: The perceived difference in outcomes between cystic and solid vestibular schwannomas cannot be demonstrated when confounding factors such as tumor size are taken into account.
Keywords: Vestibular schwannoma, facial nerve, outcome measures
Cystic appearances on magnetic resonance (MR) scanning of vestibular schwannomas (VS) are not uncommon.1,2 The incidence of cyst formation has been estimated at anything between 4%3 and 48%2 depending on the criteria used. In our unit we have identified 86 patients, from a total of 897 undergoing surgery for VS, who had tumors. This represents 9.6% of the total and is similar to the majority of reported series.4,5,6,7 Cystic tumors are thought to display more rapid growth and therefore shorter duration of symptoms, and greater facial nerve involvement.4,8 It has been said that the outcome of surgery in cystic VS is worse than that seen in solid tumors5,9 as far as facial nerve function is concerned. As there have been conflicting views on this matter, the present study sought to determine explicitly whether postsurgical facial nerve outcomes for patients with a cystic component to a VS were significantly different from those with a solid tumor.
METHODS
We examined the prospectively maintained VS database, held by the skull base surgery department, and identified 86 patients who were recorded as having cystic VS and who had undergone surgery between May 1981 and the present. All of these patients had undergone surgery by one surgeon, the senior author (DAM). Of those 86 only 59 patients had records in the database showing their facial nerve outcome at 2 years following surgery. The case notes of the remaining patients were obtained and data corresponding to outcomes at 2 years were identified as far as possible. This gave a final list of 77 patients with cystic tumors who had outcome data at 2 years. Three of these had to be excluded because the records did not hold data for the size of the tumor, which was necessary for matching with the control group.
The database contained 897 patients but only 343 had clearly been identified as having a tumor that was not cystic. Seventy-seven of those 343 patients were identified who matched the 77 patients with a cystic tumor as closely as possible and who had outcome data available either from the database or, if necessary, from referral to hospital records. They were matched according to surgical approach, facial nerve grade preoperatively, tumor size, and age. These factors were chosen following the findings of a paper by Gray et al.10 Facial nerve function was graded using the House-Brackmann system.11 The tumor size was stratified as less than 1.5 cm, 1.5 to 2.4 cm, 2.5 to 3.4 cm, 3.5 to 4.5 cm, or greater than 4.5 cm for maximum tumor diameter axially mediolaterally in the plane of the internal auditory canal.
In a small number of cases the facial nerve had been lost during surgery and was not repaired. Those who had House-Brackmann scores less than 6 due to static facial procedures or hypoglossal-facial anastamosis were recorded for the purposes of this study as grade 6.
As the data are ordinal and not normally distributed, the Wilcoxon signed rank test for paired data was used to analyze it using R version 2.3.012 on Mac OS X 10.4.6 (Apple, Inc., Cupertino, CA).
RESULTS
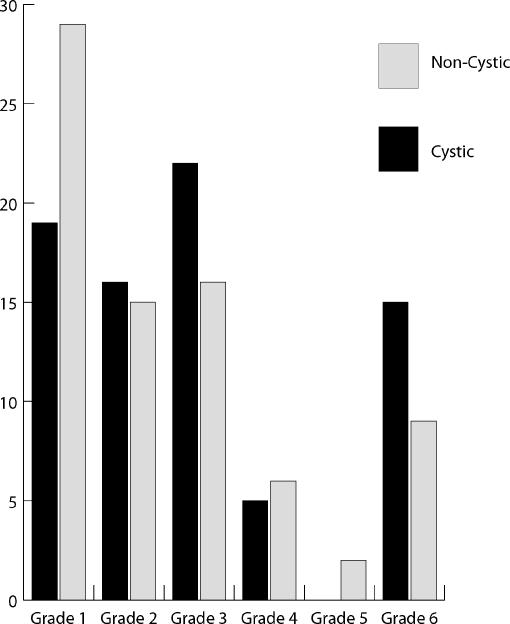
The results of House-Brackmann scores at 2 years after surgery are summarized in Fig. 1.
Figure 1.
House-Brackmann scores at 2 years according to tumor type.
The p value for the Wilcoxon signed rank test was .0997. Therefore no statistically significant difference could be found between the two groups.
Clearly this may be an issue with the power of this study and so a type 2 error must be excluded. We calculated the sample size needed for the study in the same manner as for parametric data based on a power of 0.9 and significance level of 0.05 and looking for a difference in House-Brackmann scores of 1. A correction factor of 15% was then used, based on the asymptotic relative efficiency of the Wilcoxon signed rank test compared with the t-test. This gives a final estimate of the required sample size of 70.04. Our sample size was 77 patients in each of the two groups. The study therefore does have significant power to answer the question to this degree of significance.
This result is also supported by the 95% confidence interval for the data which is −5.645622 × 10−5 to 1.499988. This interval includes 0 and therefore suggests that the true result is nonsignificant.
DISCUSSION
In our study, we compare the outcomes of tumors of similar size and so avoid size as a confounding factor. Of the previous studies suggesting worse outcomes for cystic tumors, some authors4 have not done this and have simply looked at the raw outcomes of patients with cystic tumors. In fact, in the study by Charabi et al, the median size of the tumors was 45 mm. As cystic VS are thought to display faster growth3 and therefore to present with larger tumors, this may be one reason why they are thought to be the cause of worse facial nerve function.
Different studies have used different inclusion criteria for tumors to be labeled as cystic. In the article by Charabi4 he describes three criteria: the presence of hypodense/isointense areas within the solid isodense/isointense portion of the tumor, or in association to it, on, respectively, computed tomography or MR images; the per-operative identification of cystic elements; and histological verification, with demonstration of the presence of S-100 positive membranous structures in the tumors. This may be why he has such a low incidence of cyst formation in his series. Zaouche et al9 and Deguine et al13 identified cystic tumors on the basis of a T1-weighted MR imaging sequence with gadolinium infusion by irregular contrast enhancement or by the presence of areas of different density (or signal intensity) inside the tumor. In our series, tumors were classified as cystic by the senior author (DAM) according to definitive radiological investigation of the day and on per-operative observation, but we did not include any histological criteria.
CONCLUSION
We have not been able to demonstrate any significant difference in facial nerve outcomes between cystic and noncystic tumors. This is not due to a type 2 error, as was demonstrated above. The similar outcomes are therefore real and, we believe, good evidence that one should not expect worse facial nerve outcome following the removal of cystic VS. We feel that the presence of a cystic vestibular per se is not a cause of postoperative facial nerve palsy. Of the other studies we have identified, most appear to be based on the same 23 patients (Charabi) and do not look for confounding factors such as tumor size, which is a known feature of cystic VS. While it may be true that cystic VS is associated with poorer facial nerve outcome following surgery, we feel that this is due to their size.
Several studies have suggested that cystic tumors are unsuitable for management by observation with serial scanning when identified at an early stage, due to their expected rapid growth rate.4,5 We would agree with this statement as our results would suggest that patients undergoing surgery at this stage should have facial nerve function as good as that caused by noncystic tumors.
ACKNOWLEDGMENT
Thanks to Dr. A.T. Prevost of the Medical Research Council Biostatistics Unit, University of Cambridge, for his advice on the statistical analysis.
REFERENCES
- Wallace C J, Fong T C, Auer R N. Cystic intracranial schwannoma. Can Assoc Radiol J. 1993;44:453–459. [PubMed] [Google Scholar]
- Jeng C M, Huang J S, Lee W Y, et al. Magnetic resonance imaging of acoustic schwannomas. J Formos Med Assoc. 1995;94:487–493. [PubMed] [Google Scholar]
- Charabi S. Acoustic neuroma/vestibular schwannoma in vivo and in vitro growth models. A clinical and experimental study. Acta Otolaryngol Suppl. 1997;530:1–27. [PubMed] [Google Scholar]
- Charabi S, Tos M, Borgesen S E, Thomsen J. Cystic acoustic neuromas. Results of translabyrinthine surgery. Arch Otolaryngol Head Neck Surg. 1994;120:1333–1338. doi: 10.1001/archotol.1994.01880360031006. [DOI] [PubMed] [Google Scholar]
- Fundová P, Charabi S, Tos M, Thomsen J. Cystic vestibular schwannoma: surgical outcome. J Laryngol Otol. 2000;114:935–939. doi: 10.1258/0022215001904653. [DOI] [PubMed] [Google Scholar]
- Wandong S, Meng L, Xingang L, et al. Cystic acoustic neuroma. J Clin Neurosci. 2005;12:253–255. doi: 10.1016/j.jocn.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Tali E T, Yuh W T, Nguyen H D, et al. Cystic acoustic schwannomas: MR characteristics. AJNR Am J Neuroradiol. 1993;14:1241–1247. [PMC free article] [PubMed] [Google Scholar]
- Lanser M J, Jackler R K, Pitts L H. In: Tos M, Thomsen J, editor. Acoustic Neuroma. 1st ed. Amsterdam, NY: Kugler Publications; 1992. Intratumoral hemorrhage and cyst expansion as causes of acute neurological deterioration in acoustic neuroma patients. pp. 229–234.
- Zaouche S, Ionescu E, Dubreuil C, et al. Pre- and intraoperative predictive factors of facial palsy in vestibular schwannoma surgery. Acta Otolaryngol. 2005;125:363–369. doi: 10.1080/00016480410025216. [DOI] [PubMed] [Google Scholar]
- Grey P L, Moffat D A, Palmer C R, et al. Factors which influence the facial nerve outcome in vestibular schwannoma surgery. Clin Otolaryngol. 1996;21:409–413. doi: 10.1046/j.1365-2273.1996.00816.x. [DOI] [PubMed] [Google Scholar]
- House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- R Development Core Team R a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2005. Available at: http://www.R-project.org Available at: http://www.R-project.org
- Deguine O, Maillard A, Bonafe A, et al. Pre-operative and peri-operative factors conditioning long-term facial nerve function in vestibular schwannoma surgery through translabyrinthine approach. J Laryngol Otol. 1998;112:441–445. doi: 10.1017/s0022215100140733. [DOI] [PubMed] [Google Scholar]