Abstract
Chronic cocaine administration typically results in increased locomotor activity, known as behavioral sensitization. Investigating the time course of locomotor activity across trials may provide a more detailed analysis of the temporal changes that might occur within sensitization. Prior research with rodents shows that the peak of locomotor activity shifts from acute to chronic drug administration. The purpose of the current experiment was to investigate acute versus chronic cocaine effects on locomotor activity in an avian species, Japanese quail, and to investigate whether this phenomenon is dose dependent. Subjects received daily ip injections of saline or 5, 10, or 20 mg/kg cocaine for 20 days. Following each injection, birds were placed in standard locomotor activity chambers, and activity was recorded for 150 min. A cocaine challenge was given after a ten-day withdrawal period. Two retraining trials were given to reestablish cocaine responding prior to a saline challenge in the drug-paired environment. Results showed that repeated administration of the 10 mg/kg dose of cocaine enhanced activity across 120 min compared with acute administration. In contrast, repeated administration of the 20 mg/kg dose resulted in greater cocaine-induced activity for 60 min compared with acute administration. In addition, behavioral sensitization was shown to be dose-dependent and appeared to be due, at least in part, to conditioning.
Keywords: Avian, Birds, Behavioral sensitization, Temporal effects, Cocaine, Dose-dependent effect, Contextual conditioning
1. Introduction
Repeated intermittent administration of psychostimulants often results in behavioral sensitization [1,2]. Behavioral sensitization is typically identified as an increase in locomotor activity or stereotypic behaviors as a result of chronic administration of a psychostimulant [3,4,5]. Even though chronic psychostimulant administration may result in an overall increase in behavioral activity, the pattern of activity across time may implicate other drug mechanisms, such as tolerance, that may occur concurrently with behavioral sensitization. Few researchers have investigated the temporal pattern of drug effects on locomotor activity and among those that have, the focus has been on investigating changes in the peak of locomotor activity from acute to chronic drug administration. For example, Post and Rose [6] gave rats an injection of either saline or cocaine (10 mg/kg intraperitoneally or ip) and monitored locomotor activity and stereotypic behaviors for 90 min, once a day for 12 days. Acute cocaine administration resulted in a peak of locomotor activity early in the session (around 15 min), whereas chronic administration resulted in a later onset of peak activity (around 90 min). Thus, a rightward shift in peak locomotor activity was found from acute to chronic administration of cocaine.
Ansah et al. [7] replicated this experiment except that rats were given a higher dose of cocaine (20 mg/kg ip) and locomotor activity was monitored for 3 hr, once a day for 30 days. Similar to the temporal pattern that Post & Rose [6] found, the peak in locomotor activity was shifted rightward. On Day 1, locomotor activity peaked at 15 min after drug administration. By Day 12, the peak of the cocaine-induced response had shifted to 95 min.
The purpose of the current experiment was to characterize the temporal pattern of locomotor activity from acute to chronic cocaine administration in an avian species, male Japanese quail. Although rodents have been the primary species used in drug addiction research (for a review, see Bardo, [8]), they typically do not have good visual acuity and tend to rely on multimodal cues in their environment. Since humans depend on visual sensory information, a model of human drug dependence that consists of a more visually-oriented animal may be of additional relevance to human drug addiction. Japanese quail have a well-developed visual system [9] and demonstrate innate and learned preferences for color [10,11,12]. In addition, cocaine-induced behavioral sensitization has previously been demonstrated in aves [13], as well as cocaine reward [14,15,16]. In the present experiment, visual cues were presented with a drug such that visually salient cues could become associated with a drug state.
In addition to investigating temporal characteristics of cocaine-induced locomotor activity, we were interested in the dose-dependent characterization of cocaine on locomotor activity in this species. Dose-dependent effects on cocaine-induced locomotor activity have been well documented in rodents [17] but have not been reported in avian species that better utilize visual cues. In the present experiment, male Japanese quail were given either saline or 1 of 3 doses of cocaine, 5, 10, or 20 mg/kg, once a day for 20 days and locomotor activity was examined for 150 min after each injection. To determine the role of the conditioning in cocaine locomotor-activating effects, two additional tests were given after conditioning. First, a saline challenge was administered in the context previously paired with cocaine. Second, cocaine was administered in a novel context.
2. Material and methods
Subjects
Thirty-two male Japanese quail (Coturnix japonica) approximately four months-old served as subjects. Quail were hatched (from eggs acquired from GQF Manufacturing; Savannah, GA) and raised together in a brooder until sexual maturity that occurred at approximately 28 days of age. At 28 days of age, males were individually housed in metal cages (50.8 × 25.4 × 21.4 cm) and were maintained on a 16:8 light/dark schedule with food and water available ad libitum. All of the experimental procedures were conducted under the guidelines of the Division of Laboratory Animal Research at the University of Kentucky. The Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985) were followed.
Drugs
Cocaine hydrochloride (National Institute for Drug Abuse; Bethesda, MA), was mixed in mammalian physiological saline (0.9%) and injected intraperitoneally (ip) at a volume of 3 ml/kg body weight. Mammalian physiological saline has been used in previous experiments that have demonstrated cocaine effects in birds [e.g., 14]. All doses are expressed as the salt weight. The experiment utilized three doses of cocaine 5, 10, and 20 mg/kg. Mean body weight for subjects that received cocaine was approximately 150 g throughout the experiment.
Apparatus
Sixteen standard locomotor activity chambers (28.6 long × 21.2 wide × 21.2 cm deep; Med Associates; Georgia, VT) were used to quantify locomotor activity. All chambers had wire mesh floors covered with brown lightly-textured paper, clear ceilings, and green and yellow alternating stripes in either a vertical or horizontal orientation on the walls. Each chamber had six photobeams that were approximately 6.4 cm apart and 3.2 cm above the floor. A Med Associates program (Georgia, VT) was used to collect photobeam breaks in five-min increments.
Procedure
Prior to receiving any injections, birds were given a one day habituation period (150 min; the same length as training trials) in their assigned locomotor chamber. Following habituation, birds received 20 training trials, one per day. Training trials consisted of an ip injection of saline or 5, 10, or 20 mg/kg cocaine and placement into the locomotor chamber for 150 min. Groups were designated as S, C5, C10, and C20, respectively (n's = 8). After the last training trial, birds were housed in their home cages for a ten-day withdrawal period. The next day, birds that previously received cocaine were given a cocaine challenge that consisted of half of the original training dose. Half of the dose was given because giving half of the original dose is a more conservative test for investigating drug effects. Birds that received saline during training were given an injection of saline. Although it is more common to inject the saline control group with cocaine and compare the response with that of groups that were previously exposed to cocaine, others have investigated drug effects by giving the saline control group saline rather than cocaine [18,19]. After the challenge, the following sequence of training and test days were conducted: two re-training trials; a saline challenge in the cocaine-paired chamber; two re-training trials; and a cocaine challenge in a novel chamber. Re-training trials followed the same procedure as the first 20 training trials and the same dose of cocaine was administered as during training trials. Retraining trials were intended to reestablish cocaine-induced locomotor effects. The cocaine challenge administered in the novel context consisted of an injection of half of the original training dose of cocaine. Just as in the first cocaine challenge, the saline group was given saline.
During training, half of the birds that received cocaine were trained in a context with yellow and green horizontal stripes, while the other half were trained in a context with yellow and green vertical stripes. During the final cocaine challenge, birds received cocaine and placement into the chamber that contained a novel orientation of the yellow and green stripes. White noise was present during all trials.
Statistical Analyses
Two-way repeated-measures Analyses of Variance (ANOVAs) were performed on the frequency of photobeam breaks in 15 min time bins for each group (dose) separately. This was followed by planned comparisons at each time bin to determine differences between acute and chronic cocaine effects on locomotor activity. To investigate dose-dependent effects, mean photobeam breaks for each group for the total 150 min was compared in a one-way ANOVA. A Fischer's Protected LSD post-hoc analysis was used to determine further differences between groups. Because the typical trial length that is used in rodent experiments to determine locomotor effects of psychostimulant drugs is 60 min or less [e.g., 20,21,22], we also analyzed the first 60 min of locomotor activity. To analyze the saline and final cocaine challenge for conditioning effects, we performed repeated-measures ANOVAs comparing the average of the retraining trials with training trial 20. Once it was established that retraining resulted in responding similar to the last training trial, one-way ANOVAs followed by Fischer's Protected LSD post-hoc analyses were conducted, where appropriate, to determine group (dose) differences during a saline challenge, and to determine group differences when cocaine was administered in a novel chamber.
All descriptive statistics are presented as mean ± SEM (standard error of the mean) and the significance level was set at p ≤ 0.05. Data from one bird that had been assigned to the 10 mg/kg group were not included in the analyses due to missing data during training.
3. Results
The temporal pattern of acute and chronic cocaine effects on locomotor activity was investigated by collapsing the data into 15 min time bins and performing a repeated-measures ANOVA on trials 1, 10, and 20 separately for each group. Figure 1a (top panel) represents mean photobeam breaks across 15-min time bins for trials 1, 10, and 20 for the saline group. The saline group showed increased locomotor activity toward the end of the first trial. By trials 10 and 20, this pattern of activity was no longer evident. This resulted in a significant time × trial interaction, F(18, 126)=2.46. There were no significant main effects of trial or time, F's<1.36. Post-hoc analyses for each trial revealed a significant main effect of time for trial 1, F(9,63)=4.62 but there was no significant effect of time for trials 10 or 20, F's(9,63)<1.49. Thus, activity appeared to change for the saline group across time on trial 1. This may have been the result of the initial novelty of the testing situation or habituation. Planned comparisons at each 15 min time bin did not indicate differences between acute and chronic trials at any of the time bins.
Figure 1.
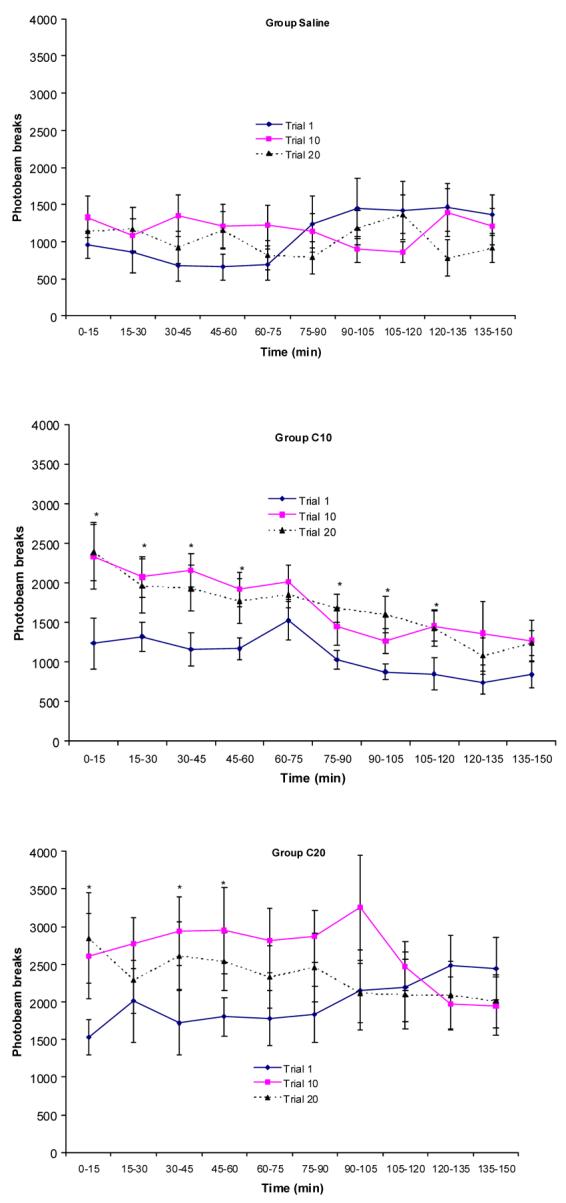
Figure 1a Mean photobeam breaks (±SEM) across 15 min time bins during trials 1,10, and 20 for the saline group (n=8). * indicates significant difference between trials for that dose at that time bin
Figure 1b Mean photobeam breaks (±SEM) across 15 min time bins during trials 1,10, and 20 for Group C10 (n=8). * indicates significant difference between trials for that dose at that time bin
Figure 1c Mean photobeam breaks (±SEM) across 15 min time bins during trials 1,10, and 20 for Group C20 (n=8). * indicates significant difference between trials for that dose at that time bin
The frequency of photobeam breaks across 15 min time bins for trials 1, 10, and 20 for group C10 is shown in Figure 1b (middle panel). Group C10 had greater locomotor activity during trial 10 compared to trial 1, as indicated by a significant main effect of trial, F(2, 14)=9.08 and appropriate post-hoc analyses. There was also a significant main effect of time, F(9, 126)=6.45, suggesting that activity started high at the beginning of each trial and later decreased across time during all of the trials. There was no significant time × trial interaction, F(18, 126)=0.44. However, planned comparisons at each 15 min time bin indicated that chronic cocaine (10 and 20 days) resulted in increased activity compared to acute cocaine (1 day) from 0 to 120 min except between 60 and 75 min.
Figure 1c (bottom panel) depicts the frequency of photobeam breaks across 15 min time bins for group C20 on trials 1, 10, and 20. A repeated-measures ANOVA indicated a significant trial × time interaction, F(18,126)=1.92. Planned comparisons at each 15 min time bin revealed significantly greater locomotor activity for trials 10 and 20 at times 0-15, 30-45, and 45-60 min, F's (2,14)>4.02. Further analyses indicated that this was due to greater activity during trials 10 and 20 during these time bins, F's (1,7)>7.3.
Figure 2 illustrates the dose response curve of mean photobeam breaks averaged across trials. The group that received 20 mg/kg of cocaine had greater locomotor activity than the other groups. A one-way ANOVA revealed a significant main effect of group, F(3,27) = 7.22. Fisher's Protected LSD confirmed that group C20 had significantly greater activity than the other groups but that no other groups differed from each other.
Figure 2.
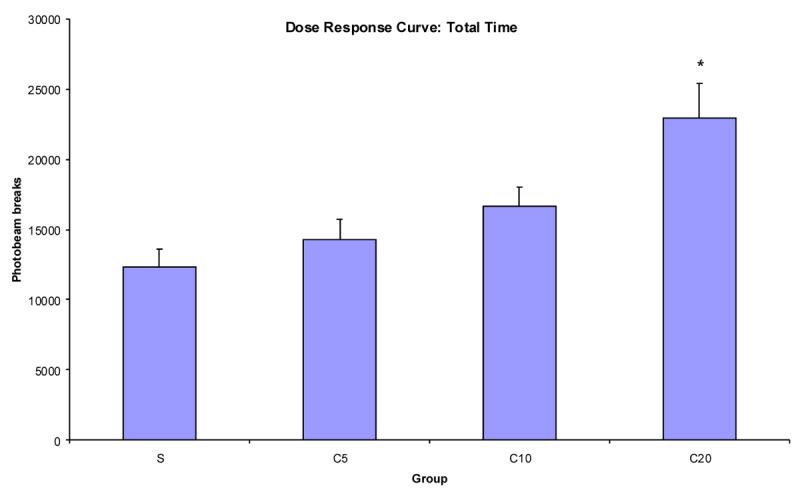
Mean photobeam breaks (+SEM) for groups S, C5, C10, and C20 (n's = 8,7,8,8, respectively) as a function of dose during the total 150 min collapsed across trial. * indicates significant difference from saline
Previous investigations of cocaine effects on locomotor activity with rodents have typically utilized a trial length of 60 min or less [20,21,22]. Figure 3 represents the mean photobeam breaks for the first 60 min of the 150 min trial for groups that received saline, or 5, 10, or 20 mg/kg cocaine. An ANOVA revealed a significant main effect of group (dose) and Fischer's Protected LSD post-hoc analysis showed that group C20 had greater locomotor activity than all of the groups and that group C10 had greater activity than the saline group. There were no other differences between groups.
Figure 3.
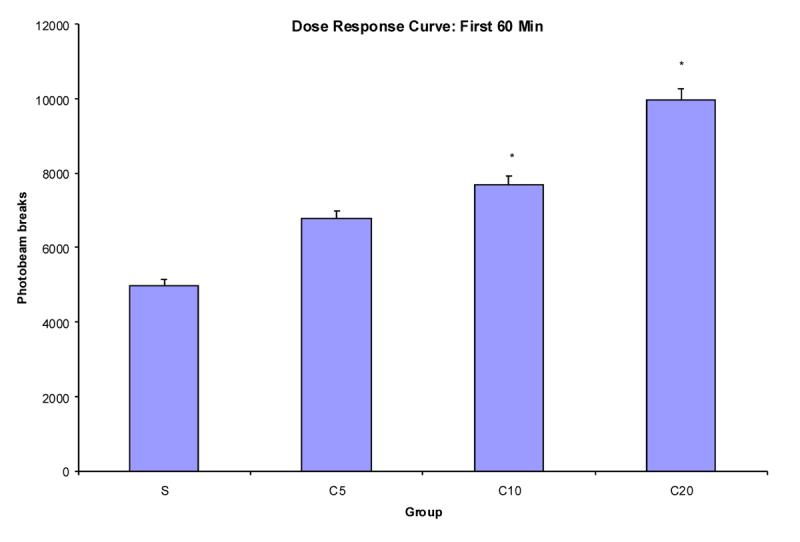
Mean photobeam breaks (+SEM) for groups S, C5, C10, and C20 (n's = 8,7,8,8, respectively) as a function of dose during the first 60 min of the 150 min trial collapsed across trial. * indicates significant difference from saline
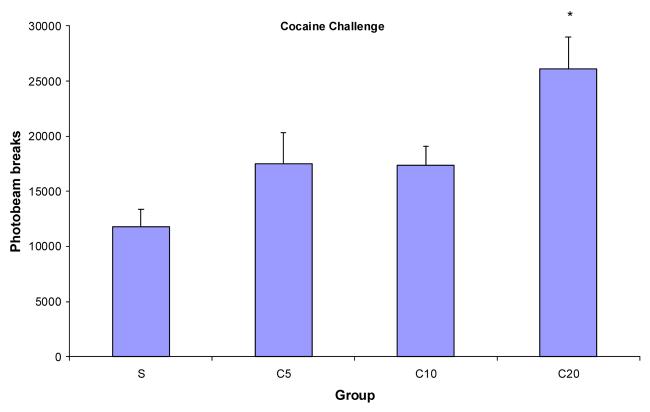
To investigate the occurrence and relatively long-lasting effects of cocaine on locomotor activity, a cocaine challenge of half of the training dose was given ten days after training. Figure 4 shows the mean photobeam breaks for the groups that were trained with saline or 5, 10, or 20 mg/kg of cocaine. During the test for sensitization, all of the groups received an ip injection of cocaine except for the saline group which received saline. Results showed that the group that received the 20 mg/kg dose of cocaine had significantly greater locomotor activity than the other groups. A one-way ANOVA revealed a significant main effect of group, F(3, 27)=6.68. A Fischer's Protected LSD post-hoc analysis indicated that group C20 had greater locomotor activity than group C10, group C5, and the saline group. None of the other groups differed from each other.
Figure 4.
Mean photobeam breaks for groups S, C5, C10, and C20 (n's = 8,7,8,8, respectively) during the cocaine challenge (half of the training dose). * indicates significant difference from saline
To test for a conditioned locomotor response, the average of the two re-training trials was compared to the saline challenge. First the average of the two re-training trials was compared to training trial 20 at each dose to ascertain whether the training level of locomotor activity had been re-established. There was no main effect of trial, F(1,27)=3.63, allowing for further comparison between the re-training trials and the saline challenge. Means(±SEM) photobeam breaks were 17718±1451 for the average of the first two training trials and 15992±1385 photobeam breaks for trial 20 of training.
Figure 5 represents the frequency of mean photobeam breaks during the total 150 min for all groups for the average of re-training trials 1 and 2 and the saline challenge that was given in the chamber previously paired with cocaine. None of the groups showed systematic increases or decreases from the re-training trials to the saline challenge. A repeated-measures ANOVA indicated that there was a significant main effect of group F(3,27)=3.19 but not trial, F(1,27)=2.78. The group × trial interaction was also not significant, F(3,27)=0.71.
Figure 5.
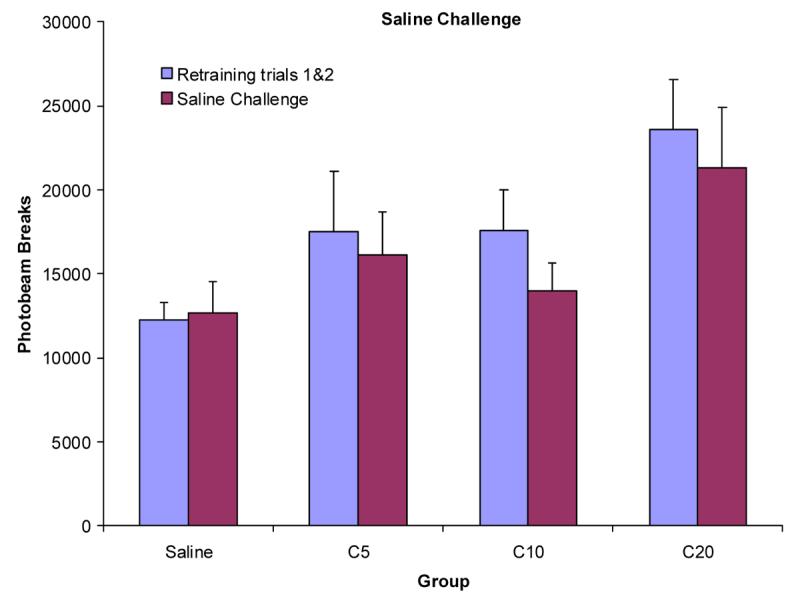
Mean frequency of photobeam breaks (+SEM) for groups S, C5, C10, and C20 (n's = 8,7,8,8, respectively) for the average of the previous re-training trials compared to the saline challenge.
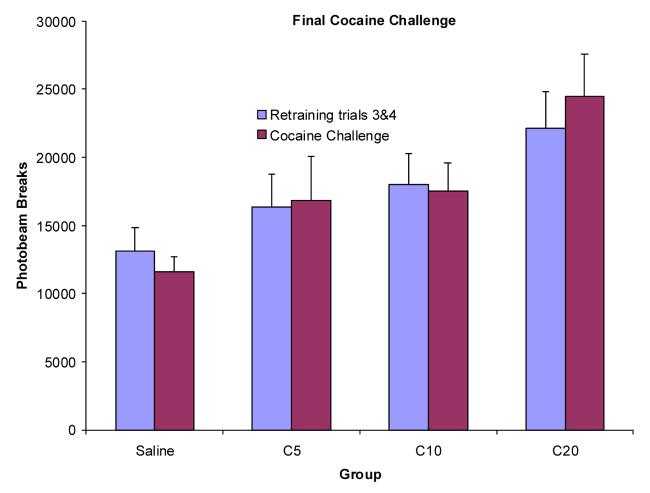
In addition to a saline challenge, a final cocaine challenge was administered in a novel chamber to test for a conditioned locomotor response. The average of two re-training trials given just before the final cocaine challenge (retraining trials 3 and 4) was not found to be significantly different from training trial 20, F(1,27)=2.51. The average of the re-training trials was then compared to the cocaine challenge that was given in the novel chamber (see Figure 6). A repeated-measures ANOVA indicated that there was a main effect of group, F(3,27)=3.83, but not of trial, F(1,27)=0.11 nor was the group × trial interaction significant, F(3, 27)=2.22. Therefore, the same dose-response pattern was evident as during the previous retraining trials but there was no change in locomotor activity from the two re-training trials to the cocaine challenge when cocaine was administered in a novel chamber.
Figure 6.
Mean photobeam breaks (+SEM) for groups S, C5, C10, and C20 (n's = 8,7,8,8, respectively) for the average of the retraining trials compared to the cocaine challenge (half of the training dose) in the novel context.
4. Discussion
The results of the analyses of the temporal pattern of locomotor activity indicated differences between acute and chronically-treated birds and that these differences depended on dose. Locomotor activity was enhanced for the majority of the first 60 min in birds given 10 and 20 injections of a 20 mg/kg dose of cocaine compared to 1 injection of the 20 mg/kg dose. Yeh and Haertzen [23] found similar results with rodents. They gave a 20 mg/kg dose of cocaine and found that horizontal activity in the chronically-treated cocaine rats (15 injections) was significantly increased during the first 60 min compared with acute administration (1 injection). In addition, in the current experiment, birds that were given 10 and 20 injections of a 10 mg/kg dose of cocaine had significantly greater locomotor activity for the majority of the first 120 min compared to that of birds injected once with this dose.
This dose-dependent difference between acute and chronic cocaine-induced locomotor activity across time may have important implications for the use of this species in future investigations of drug abuse. Of particular interest is that repeated administration of a 10 mg/kg dose of cocaine enhanced locomotor activity for 120 min. The literature with rodents has demonstrated that repeated cocaine alters locomotor activity between 60 and 95 min with doses of cocaine as high as 20 mg/kg (e.g., [6,7,23]). It has been well-established that avian metabolism is higher than that of mammals [24]. Therefore, the results of the current temporal data are a bit puzzling if metabolism in birds is similarly correlated with the behavioral effects of drugs, as it appears to be in rodents. Unfortunately, there are no psychopharmacological or molecular data indicating that the action of cocaine might differ in birds compared with that of mammals.
There was some evidence for cocaine sensitization in the 20 mg/kg group. The 20 mg/kg group showed greater cocaine-induced responding than the saline group when the dose response curve was examined, and had greater cocaine-induced responding than the other groups during a cocaine challenge. Our methodology of not administering cocaine to the saline control group makes the interpretation of the cocaine challenge data less clear. However, groups 5 and 10 (groups that received 5 and 10 mg/kg of cocaine) also did not differ from saline, making it unlikely that one injection of a low dose of cocaine would have resulted in greater responding in the saline group. Therefore, our results would have not likely been different if we had given the saline group cocaine.
The temporal data provide additional information about birds that received repeated administration of a 20 mg/kg dose of cocaine. They were not only sensitized compared to the other groups but according to the temporal data, repeated administration of a 20 mg/kg dose of cocaine only enhanced locomotor activity above acute administration for the first 60 min. This is in comparison to a 10 mg/kg dose that enhanced locomotor activity for the first 120 min. Tolerance is typically observed when high continuous doses of cocaine are used (e.g., 40 mg/kg /day; [25]) and is customarily represented as a rightward shift in the dose-dependent relationship or a downward shift compared to saline controls. In the current experiment, the dampening of the drug effect on locomotor activity across time for the 20 mg/kg dose compared to the 10 mg/kg dose may represent the emergence of a behavior that competes with locomotor activity such as stereotypy. Stereotypy-like behavior has been observed in pigeons as apomorphine-induced pecking but the pecking has to be elicited by patterned stimuli on the walls [26]. Such pecking behavior has not been observed anecdotally in any of our cocaine studies. Alternatively, the dampening represents desensitization to cocaine's locomotor-activating effects or tolerance. Tolerance to cocaine has been demonstrated in pigeons using a variable-dosing procedure in which key pecking was maintained under a fixed-ratio schedule of reinforcement [27,28]. Although our interpretation of this finding is tentative, it provides possible evidence of tolerance to cocaine's locomotor activating effects in an avian species.
The current findings indicate that conditioning might play a role in sensitization. In the current experiment, quail were challenged with a saline injection in the environment that was previously paired with cocaine or saline for the saline control. It was predicted that, if conditioning to the environment had occurred, birds that were previously trained with cocaine in the environment would show a similar or greater response to a saline injection in that environment. In other words, the cocaine-paired environment should be predictive of receiving cocaine if it had become excitatory from being paired with the drug state. Results showed that none of the birds demonstrated a significant change in locomotor responding from the re-training trials in which they were given cocaine in the context to the saline injection in the same context. This suggests that, at least part of the response to cocaine may have been specific to the context or environment in which cocaine was given.
To further assess whether cocaine-induced responding was specific to the context, birds were retrained to training levels and a cocaine challenge was administered in a novel context. Cocaine-induced locomotor activity did not change for any of the groups from retraining levels when cocaine was administered in a novel context. If cocaine-induced responding had been context-specific, a decrease in responding would be evident when cocaine was given in a novel context. It is unclear why our saline challenge suggested that conditioning might have been involved in the cocaine-induced response but that our cocaine challenge in a novel environment did not. One possibility is that the contexts were not distinct enough for the birds to discriminate. However, previous findings from our laboratory indicate that male quail readily discriminate between these two wall patterns by demonstrating a place preference for the context in which they received cocaine over the context in which they received saline [14]. An alternative explanation is that the novelty of the context where birds were tested with cocaine during the last cocaine challenge resulted in increased activity due to stress and this was additive to the physiological effect of cocaine. Previous research suggests that quail demonstrate neophobia to novel situations [29]. In contrast to rodents, quail tend to demonstrate increased locomotor activity to neophobic situations. Another possibility is that quail most likely were using the context and the discriminative stimulus properties of cocaine. Further experimentation with an alternative procedure may be necessary to parse out conditioning effects.
In sum, the findings provide novel evidence for cocaine's effect on locomotor activity in an avian species. Cocaine alters locomotor activity in birds across time and it does so dose-dependently. A relatively smaller dose of cocaine had longer lasting effects (120 min) during chronic administration than acute administration. The effects of a higher dose of cocaine on locomotor activity were only evident during the first 60 min. This same dose resulted in behavioral sensitization and therefore it is possible that sensitization and tolerance were occurring simultaneous. The findings also provide tentative evidence for the possible role of context-specificity. In general, the findings of the current experiment indicate that the use of an avian species in investigating drug effects may provide information of additional relevance to human drug abuse.
Acknowledgements
The authors gratefully acknowledge Robert Prather, Brad Presley, and Shiraz Yazdani for help with data collection. This research was supported by USPHS grant K01-DA00508 (awarded to C.K. Akins) and a Research Challenge Trust Fund fellowship by the University of Kentucky (awarded to E.H.Geary). We would also like to thank NIDA for providing the cocaine used in this experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–6. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 2.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Rev. 2003;41:203–28. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 3.Churchill L, Kalivas PW. The involvement of the mediodorsal nucleus of the thalamus and the midbrain extrapyramidal area in locomotion elicited from the ventral pallidum. Behav. Brain Res. 1999;104:63–71. doi: 10.1016/s0166-4328(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 4.Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J. Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 5.Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 1998;60:593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 6.Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–2. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- 7.Ansah TA, Wade LH, Shockley DC. Changes in locomotor activity, core temperature, and heart rate in response to repeated cocaine administration. Physiol. Behav. 1996;60:1261–67. doi: 10.1016/s0031-9384(96)00250-8. [DOI] [PubMed] [Google Scholar]
- 8.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit. Rev. Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith TH. Optimization, constraint and history in the evolution of eyes. Q. Rev. Biol. 1990;65(3):281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- 10.Kovach JK. Binomial assessment of behavioral-phenotypic variations: constancy of choices, trial effects, and social interaction effects in mass-screened color preferences of quail chicks (Coturnix coturnix japonica) J. Comp. Physiol. Psychol. 1977;91:851–7. doi: 10.1037/h0077357. [DOI] [PubMed] [Google Scholar]
- 11.Kovach JK. Visual information and approach behavior in genetically manipulated quail chicks: preference hierarchies and interactions of flash rate, flash amplitude, luminance, and color. J. Comp. Physiol. Psychol. 1980;94:178–99. doi: 10.1037/h0077642. [DOI] [PubMed] [Google Scholar]
- 12.Kovach JK. Constitutional biases in early perceptual learning: I. Preferences between colors, patterns, and composite stimuli of colors and patterns in genetically manipulated and imprinted quail chicks (C. coturnix japonica) J. Comp. Psychol. 1983;97:226–39. [PubMed] [Google Scholar]
- 13.Levens N, Akins CK. Chronic cocaine pretreatment facilitates Pavlovian sexual conditioning in male Japanese quail. Pharmacol. Biochem. Behav. 2004;79:451–7. doi: 10.1016/j.pbb.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Akins CK, Levens N, Prather R, Cooper B, Fritz T. Dose-dependent cocaine place conditioning and D1 dopamine antagonist effects in male Japanese quail. Physiol. Behav. 2004;82:309–15. doi: 10.1016/j.physbeh.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA, Baker MR, Rettig KM. Cocaine-conditioned place preference in young precocial domestic fowl. Exp. Clin. Psychopharmacol. 1995;3:105–111. [Google Scholar]
- 16.Levens N, Akins CK. Cocaine induces conditioned place preference and increases locomotor activity in male Japanese quail. Pharmacol. Biochem. Behav. 2001;68:71–80. doi: 10.1016/s0091-3057(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 17.O'Dell LF, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Pharmacol. 1996;123:144–53. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- 18.Jayaram P, Steketee JD. Cocaine-induced increases in medial prefrontal cortical GABA transmission involves glutamatergic receptors. Eur. J. of Pharmacol. 2006;531:74–9. doi: 10.1016/j.ejphar.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Tirelli E, Michel A, Brabant C. Cocaine-conditioned activity persists for a longer time than cocaine-sensitized activity in mice: Implications for the theories using Pavlovian excitatory conditioning to explain the context-specificity of sensitization. Behav. Brain Res. 2005;165:18–25. doi: 10.1016/j.bbr.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Bahi A, Boyer F, Bussard G, Dreyer JL. Silencing dopamine D3-receptors in the nucleus accumbens shell in vivo induces changes in cocaine-induced hyperlocomotion. Eur. J. Neurosci. 2005;21(12):3415–26. doi: 10.1111/j.1460-9568.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 21.Harrod SB, Booze RM, Welch M, Browning E, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol. Bio. Behav. 2005;82(1):170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Todetnekopf MS, Carlezon WA. Contribution of drug doses and conditioning periods of psychomotor stimulant sensitization. Psychopharmacol. 2006;185:451–458. doi: 10.1007/s00213-005-0259-1. [DOI] [PubMed] [Google Scholar]
- 23.Yeh SY, Haertzen CA. Cocaine-induced locomotor activity in rats. Pharmacol. Biochem. Behav. 1991;39:723–7. doi: 10.1016/0091-3057(91)90154-t. [DOI] [PubMed] [Google Scholar]
- 24.Dawson WR, Whittow GC. Regulation of body temperature. In: Whittow GC, editor. Sturkie's avian physiology. Academic Press; San Diego, CA: 2000. pp. 344–379. [Google Scholar]
- 25.Izenwasser S, French D. Tolerance and sensitization to the locomotor-activating effects of cocaine are mediated via independent mechanisms. Pharmacol. Biochem. Beh. 2002;73:877–82. doi: 10.1016/s0091-3057(02)00942-5. [DOI] [PubMed] [Google Scholar]
- 26.Keller S, Delius JD. Discriminative learning occasioned by the administration of a dopamine agonist. Psychopharm. 2001;157:320–323. doi: 10.1007/s002130100847. [DOI] [PubMed] [Google Scholar]
- 27.Branch MN, Wilhelm MJ, Pinkston JW. A comparison of fixed and variable doses of cocaine in producing and augmenting tolerance to it effects on schedule-controlled behavior. Beh. Pharmacol. 1968;11:1–8. doi: 10.1097/00008877-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Stafford D, Branch MN, Hughes CE. Persistence of tolerance to effects of cocaine on schedule-controlled behavior in pigeons. Behav. Pharmacol. 1994;5:581–90. doi: 10.1097/00008877-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neuro. Biobehav. Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]