Abstract
Skeletal muscle has been recognized as an essential source of progenitor or satellite cells, which are primarily responsible for muscle regeneration. Recently, muscle has also been identified as a valuable source of postnatal stem cells that appear to be distinct from satellite cells and possess the ability to differentiate into other cell lineages. These cells, named muscle-derived stem cells, possess a high myogenic capacity and effectively regenerate both skeletal and cardiac muscle. Remarkably, when genetically modified ex vivo to express growth factors, these cells can differentiate into osteogenic and chondrogenic lineages and have been shown to promote the repair of bone and cartilage. Muscle stem-cell-based regenerative therapy and tissue engineering using ex vivo gene therapy, are promising approaches for the treatment of various musculoskeletal, cardiovascular, and urological disorders.
Keywords: Muscle, Stem cell, Gene therapy, Bone tissue engineering, Cartilage tissue engineering
Combining gene therapy techniques with stem cells isolated from adult organs and tissues is becoming a very attractive strategy for the treatment of various health disorders. Tissue engineering using the body’s own cells to repair, replace, or augment diseased tissue is a rapidly evolving field. Researchers are eager to explore new sources of multipotent cells that can repopulate organ-specific progenitor and precursor cells as well as differentiate into multiple tissue types.
Skeletal muscle may constitute a convenient source of stem cells for use in cellular and cell-mediated therapies. Only a minimally invasive procedure is required to harvest the tissue via muscle biopsy. It can be safely performed in an ambulatory setting without compromising patient health. Isolated muscle-derived cells tolerate ex vivo manipulation well, and can be easily transduced with a variety of viral vectors.
Research over the past few years has shown the presence of several stem cell populations in skeletal muscle [1–3]. Satellite cells located beneath the basal lamina of mature skeletal muscle fibers, often referred as “muscle stem cells”, have long been considered monopotential stem cells capable of giving rise only to cells of the myogenic lineage. Muscle-derived stem cells (MDSCs), which may represent a predecessor of the satellite cell, are considered to be distinct in that they may not be restricted to the myogenic lineage or mesenchymal tissues and can differentiate into multiple lineages [4–7]. Among other progenitor cells found in skeletal muscle are side-population cells, mesoangioblasts, and pericytes [3, 8, 9]. The origin of these stem cell populations and their relationship to satellite cells remain largely unknown. It is unclear whether the MDSCs described in these referenced reports, and isolated via different techniques, represent the same stem cell population, or whether they represent the same population at a different stage of maturation within the myogenic lineage [2, 10].
Our research group has reported the isolation of a unique cell population from postnatal mouse skeletal muscle through the use of the preplate technique. These highly-purified muscle-derived cells exhibited long-term proliferation ability, the capacity to renew themselves, immune-privileged behavior, and multipotent differentiation in vitro and in vivo [11]. In contrast to satellite cells, these cells were able to significantly improve the efficiency of muscle regeneration and the delivery of dystrophin to dystrophic muscle in a mouse model for Duchenne muscular dystrophy.
Recently, we reported that human adult skeletal muscle contains, in addition to satellite cells and endothelial cells, a population of cells that co-express myogenic and endothelial cell markers. This population of myoendothelial cells demonstrated a superior capacity to regenerate injured skeletal muscle and underwent myogenic, chondrogenic, and osteogenic differentiation in vitro[12]. Our group and others have also reported on isolation, by flow cytometry, of human pericytes, cells associated with microvascular walls in the human skeletal muscle, that are myogenic precursors distinct from satellite cells and may be a promising candidate for future cell-therapy endeavors [3, 9, 13].
Skeletal muscle-derived cells, including stem cells, have been used to improve the healing of multiple tissues within the musculoskeletal system (muscle, bone, cartilage, ligament, meniscus), and have been employed to treat heart failure and urological dysfunction. These cells have also been used as gene delivery vehicles in both muscle-related diseases (e.g., Duchenne muscular dystrophy) and non–muscle-related diseases (e.g., hemophilia B, diabetes). We will briefly review the potential use of these cells for the treatment of bone and cartilage injuries, skeletal muscle dystrophies, and cardiovascular and urological disorders.
Bone tissue engineering
Segmental bone loss resulting from traumatic injuries, surgical tumor removal, and reconstructive operations requires void filling using bone graft substitutes. Currently available treatments, including autologous or allogenic bone grafting, allografts supplemented with demineralized bone matrix or osteogenic proteins, and bone transport or vascularized bone graft, are limited [14]. Biological failure of engraftment is common, and probably relates to a reduced osteogenic capacity of the donor bone-forming cells. Thus, induction of bone formation on a bioengineered scaffold could provide a transplantable construct or an in vivo environment to replace the structural tissue. While efforts are being made to engineer material to release osteoinductive factors in the damaged environment, the most controllable system appears to be cell-based delivery.
Our research team has extensively studied the use of muscle-derived cells (MDCs) for bone tissue engineering. Initial studies have indicated that skeletal muscle may contain osteoprogenitor cells [15, 16]. Subsequent investigation demonstrated that purified MDCs that were engineered to express BMP-2 contributed to ectopic bone formation in the hindlimb muscle [17] and elicited complete closure of a critical-sized skull defect in SCID mice [18]. Moreover, some of the donor cells containing LacZ marker gene were found to co-express β-galactosidase and osteocalcin, an osteogenic differentiation marker, indicating that these cells were able to not only deliver BMP-2, but could also differentiate toward the osteogenic lineage [15, 16]. Similar experiments performed with a clonal population of purified MDSCs demonstrated even more accelerated bone healing [4]. A total of 95% of the implanted cells were found within the newly formed bone, all of which were co-localized with osteocalcin [4]. MDSCs transduced with a retrovirus encoding BMP4 induced de novo bone formation, and eventually improved bone healing in immunocompetent mice despite the presence of an immune reaction [19]. Because they have lower immunogenicity and persist longer at the bone forming sites, MDSCs may be better cellular vehicles than primary MDCs for ex vivo gene therapy-induced bone formation [20]. Several studies demonstrated that genetically-engineered human skeletal MDCs can also induce ectopic bone formation and enhance calvarial defect healing [21, 22].
We also investigated whether a combination of multiple growth factors could benefit bone regeneration. The interaction between angiogenic and osteogenic factors using MDSCs as gene delivery vehicles for enhancement of bone formation and bone healing recently was explored by Peng et al. [23, 24]. These studies demonstrated that VEGF interacted synergistically with both BMP4 and BMP2, and promoted endochondral bone formation by increasing blood supply, recruited more mesenchymal stem cells, and accelerated cartilage resorption. However, we also observed substantially different effects caused by this interaction. VEGF did not significantly alter cartilage formation during the early phase of endochondral bone formation elicited by BMP2, but significantly enhanced this process elicited by BMP4. The greater effect of VEGF on BMP4-augmented bone formation was evidenced by the presence of a larger amount of bone in comparison to that of BMP2-induced bone formation. The higher ratio of VEGF to BMP4 was detrimental to bone healing and resulted in almost complete inhibition of bone formation, whereas the higher ratio of VEGF to BMP2 only slightly reduced bone regeneration within the calvarial defects.
Recently, we explored several new strategies to optimize engineered bone growth using genetically-modified MDSCs. One approach takes advantage of the ability to regulate therapeutic gene expression; another is intended to mimic naturally-occurring concomitant expression of growth factors and their specific antagonists during the fracture healing process. Development of a self-inactivating tet-on retroviral vector enabled us to modulate BMP4 expression in vitro and regulate bone formation in vivo [25]. However, following implantation into critical-sized calvarial defects, residual bone formation was still present without induction and consequently resulted in bone overgrowth after induction, even when reduced cell numbers were used. To eliminate this undesirable effect, MDSCs expressing BMP4 were co-implanted with the same cells engineered to express Noggin, a specific BMP antagonist. This novel technique prevented residual bone regeneration and also enabled us to grow bone that was anatomically similar to the original [26].
There is no uncertainty that the role of the scaffold is very important in bone tissue engineering, and that various delivery matrices may differently affect bone regeneration. Most of the scaffolds possess osteoconductive properties and usually require addition of osteoinductive agents (growth factors or cells engineered to secrete osteogenic protein) to induce de novo bone formation. Ordinarily, the osteogenic capacity of the cell-scaffold construct initially undergoes testing at extra-skeletal sites. We believe that, because of substantially different environmental cues, there is no direct correlation between the processes of heterotopic and orthotropic bone formation after transplantation of cell-seeded scaffolds. This means that the potential for ectopic bone formation does not necessarily translate into a capacity to heal bone defects using the same cell-scaffold composite. Previous research performed in our laboratory to regenerate bone usually employed BMP4-expressing muscle-derived cells loaded on collagen or gelatin sponges. However, regenerated bone overgrowth was frequently observed after the cell-seeded sponges were transplanted into mouse calvaria. This finding prompted us to investigate the alternative use of collagen and fibrin gels for the delivery of BMP4-expressing MDSCs to induce bone formation. Our results indicate that poorer performance of the cell-scaffold construct at the extra-skeletal site may not necessarily translate to similar efficacy following transplantation into a bone defect area. In fact, while we observed delayed ossification and a reduced amount of ectopic bone when MDSCs were delivered in fibrin gels, we surprisingly found that bone defect healing was not hindered when the same cell-fibrin composite was transplanted into mouse calvaria (manuscript in preparation). When compared to spongeous material, gel scaffolds may represent an even more suitable treatment option for plastic surgeons to repair defects in the craniofacial skeleton. One very important aspect that makes gel scaffolds so attractive is the possibility of injecting these cell-seeded gels into a defect area rather than following the route of implanting them through open wound surgery. This procedure could lower morbidity rate and may profoundly reduce the economic impact of treatment.
Cartilage tissue engineering
Articular cartilage repair represents a significant challenge in orthopaedic surgery that has propelled an intense search for productive methods to repair normal hyaline cartilage. The results of our previous studies suggest that cells residing within skeletal muscle might participate in cartilage repair. An attempt to repair osteochondral defects in a rabbit knee using skeletal muscle cells was first reported by Lee et al. [27]. Muscle biopsies obtained from New Zealand White rabbits were treated with myoblasts that were adenovirally-transduced to express IGF-I. These biopsies were press-fit into an articular cartilage defect (3 mm × 3 mm) created in a weight-bearing area. This study showed that treated defects healed more effectively than the non-treated defects 6 weeks after implantation [27]. Subsequent work by Adachi et al. [28] confirmed the feasibility of an MDC-based ex vivo gene therapy for the treatment of osteochondral defects. Retrovirally-transduced and purified MDCs were embedded in bovine type I collagen gels and cultured for 3 weeks in vitro before being transplanted into full-thickness articular cartilage defects in rabbits. Muscle cells improved the healing of the defect with an efficacy equivalent to chondrocyte transplantation, and demonstrated even better integration and type II collagen expression for up to 24 weeks. The authors of the study posited that MDCs were mainly used as a gene delivery vehicle; however they also suggested that grafts may have contained a population of stem cells capable of differentiation into chondrocytes that subsequently led to the repair of the cartilage defect [28]. Our group sought to determine whether a population of MDSCs isolated from a mouse postnatal skeletal muscle had the capacity to differentiate into chondrocytes and repair articular cartilage. Kuroda et al. performed an experiment using these cells and ex vivo BMP4 gene therapy [29]. For the first time, retrovirally-transduced MDSCs secreting BMP4 protein were shown to be capable of acquiring a chondrocytic phenotype in vitro and in vivo after transplantation into full-thickness osteochondral defects created in a rat knee. Detection of LacZ transgene expression in the repair tissues after 12 weeks indicated extended survival of the transplanted cells. Histological grading of the repaired cartilage demonstrated that BMP4-expressing MDSCs contributed to persistent repair of the osteochondral defects up to 24 weeks after surgery. Recent research performed in our laboratory indicates that suppression of VEGF expression and blockade of angiogenesis with sFlt1 (a VEGF antagonist) may enhance the expression of chondrogenic genes by MDSCs that have been retrovirally-transduced to express BMP4, which may ultimately improve regeneration of articular cartilage (unpublished data).
It is important to mention that collagen gel or fibrin sealant matrices were used in our studies to deliver and retain engineered MDSCs at the cartilage injury site. These scaffolds demonstrated no adverse effect on MDSC viability, proliferation, or differentiation in vivo. It has been shown that the final concentration of fibrinogen and thrombin in three-dimensional fibrin clots can, however, affect cell behavior [30–32]. It would be beneficial to test whether MDSC behavior in fibrin clots depends upon fibrinogen and thrombin concentration as well. The ratio of cell to gel volume is another important parameter that could affect cell proliferation and differentiation and warrants further investigation. Preliminary studies performed in our laboratory revealed the presence of higher cell counts when gel to cell ratio was 1:2, 1:3, and 1:4 (unpublished data). Recent discovery of novel methods that allow for the cultivation of cells in a controlled three-dimensional environment will bring new possibilities to perform studies of cells in a more physiologically relevant environment compared to traditional two-dimensional monolayer cultures [33].
Regeneration of skeletal and cardiac muscle
Despite recent advances in the treatment of muscular dystrophies, these disorders continue to pose a formidable challenge. A novel population of MDSCs isolated in our laboratory was shown to be capable of proliferating in vitro for an extended period of time and has been observed to differentiate into myogenic, neural, and endothelial lineages both in vitro and in vivo [11]. It has been demonstrated that immune-privileged behavior, self renewal, and multi-lineage differentiation of MDSCs correlates with improved transplantation capacity and the ability to restore dystrophin expression after transplantation into mdx mice (an animal model of Duchenne muscular dystrophy) with efficiency superior to myoblast or satellite cell transplantation [11]. We also demonstrated the feasibility of extended stem cell expansion in vitro and examined the quality of expanded cell populations before using them for cell therapy and tissue engineering applications [34]. MDSCs expanded for 200 population doublings preserved their phenotype and maintained their ability to regenerate large numbers of dystrophin-positive myofibers after transplantation into the skeletal muscle of mdx mice. The regeneration index of highly expanded MDSCs was comparable to that exhibited by freshly isolated and minimally expanded cells [34].
Cell transplantation into the heart has emerged as a potential treatment for cardiac dysfunction [35–37]. Research from our laboratory indicates that MDSCs may constitute an alternative to other myogenic cells for cardiac repair. Oshima et al. demonstrated that, in comparison with myoblasts, MDSCs implanted into infracted hearts displayed greater and more persistent engraftment, induced more angiogenesis, prevented cardiac remodeling, and significantly improved cardiac function [38]. Payne et al. reported that MDSCs can also generate dystrophin-positive myocytes within the dystrophic hearts of mdx mice after intracardiac transplantation [39]. Researchers have found large grafts consisting of numerous dystrophin-positive myocytes that mostly expressed a skeletal muscle phenotype and did not express a cardiac phenotype. A small number of donor-derived myocytes expressing a hybrid cardiac-skeletal phenotype was also located at the edge of the engraftment. This phenomenon appeared to be driven by the fusion of donor-derived cells with host cardiomyocytes [39]. A few donor-derived cells within the graft also expressed an endothelial-specific marker suggesting their contribution to the formation of blood-like structures in the heart [39].
Cell therapy for urological dysfunction
Stress urinary incontinence, which results from a weak or stretched urethral sphincter, results in involuntary leakage of urine under specific circumstances (coughing, sneezing, laughing, etc.) that increase intra-abdominal pressure. Cell-based therapy appears to be a reasonable technique to build up a deficient urethral sphincter. Lee et al. demonstrated an improvement after MDSC transplantation in an animal model of stress urinary incontinence that lasted up to 4 weeks post-surgery [40]. Periurethral injection of allogeneic MDSCs did not trigger an immune response, and was found to significantly increase the leak-point pressure in sciatic nerve-transected female rats compared to denervated animals injected with saline. Another type of urinary incontinence, overflow incontinence, is an increasingly common condition in the geriatric population, and is due to impaired detrusor muscle contractility. Research has shown that MDSCs injected into the cryo-induced injury site (bladder wall) in SCID mice produced significantly improved bladder contractility after only 1 week [41]. This study further reported that some of the donor cells differentiated into α-smooth muscle actin-expressing smooth muscle cells. In addition, researchers noticed skeletal myofibers derived from the donor cells that expressed acetylcholine receptors, which indicated the location of neuromuscular junctions, suggesting that these myofibers became innervated into the bladder [41]. Several institutions have initiated clinical trials using autologous myoblast transplantation in patients with stress urinary incontinence already. Clinical results demonstrate that incontinence and quality-of-life scores were significantly improved and lasted up to 2 years after therapy, suggesting that urinary dysfunction can be treated effectively with autologous stem cells [42, 43].
Conclusion
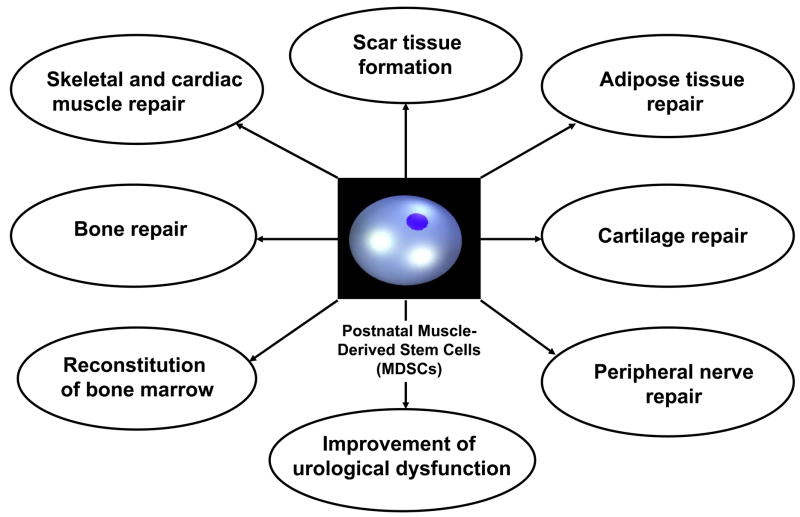
The concept of cell therapy has already been introduced into the clinical setting. Adult stem cells, particularly MDSCs, have great potential for the repair of skeletal, cardiac, and smooth muscle damage due to injury or disease (Fig. 1). Skeletal muscle also represents a valuable source for osteoprogenitor and chondroprogenitor cells that may be used in clinical practice to improve bone and cartilage healing. Clinical trials using autologous skeletal myoblast transplantation for the treatment of heart failure [44–46] and stress urinary incontinence [42, 43] are underway. The overall clinical experience indicates that stem cell therapy may be feasible in patients, can be safely performed, and is efficient if the right cell type is used in the right clinical setting. The relative ease of muscle cell isolation and purification, the cells’ tolerance for ex vivo manipulation, and cellular behaviors that were previously discussed make them very attractive for future medical therapies. More studies in our laboratory are underway to address questions and issues related to optimization of muscle-derived cell isolation and expansion, identification of various cell populations within human skeletal muscle based on established marker profiles, control of cell differentiation and transformation in different environmental cues, sexual dimorphism related to the donor cell interactions with the host, and the effects of genetic manipulation on cell therapeutic behavior. It is clear that the unrivaled potential of this therapeutic approach combined with the immediate need to answer relevant questions underlines the need for further investigation and continued basic research to elucidate the underlying mechanisms of stem cell-mediated therapies.
Fig. 1.
Adult muscle-derived stem cells (MDSCs) exhibit multipotential differentiation and have great potential for the repair of various tissues of mesodermal, endodermal, and ectodermal lineages.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27(5):924–33. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- 2.Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today. 2003;69(3):230–7. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- 3.Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–77. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150(5):1085–100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68(4–5):245–53. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 6.Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129(12):2987–95. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- 7.Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5(7):640–6. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 8.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–4. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 9.Tavian M, Zheng B, Oberlin E, Crisan M, Sun B, Huard J, et al. The vascular wall as a source of stem cells. Ann N Y Acad Sci. 2005;1044:41–50. doi: 10.1196/annals.1349.006. [DOI] [PubMed] [Google Scholar]
- 10.Bujan J, Pascual G, Corrales C, Gomez-Gil V, Rodriguez M, Bellon JM. Muscle-derived stem cells in tissue engineering: defining cell properties suitable for construct design. Histol Histopathol. 2005;20(3):891–9. doi: 10.14670/HH-20.891. [DOI] [PubMed] [Google Scholar]
- 11.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–64. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng B, Cao B, Crisan M, Sun B, Li GH, Logar A, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotech. 2007 doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 13.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 14.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55(12):1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18(6):933–44. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 16.Musgrave DS, Pruchnic R, Wright V, Bosch P, Ghivizzani SC, Robbins PD, et al. The effect of bone morphogenetic protein-2 expression on the early fate of skeletal muscle-derived cells. Bone. 2001;28(5):499–506. doi: 10.1016/s8756-3282(01)00413-6. [DOI] [PubMed] [Google Scholar]
- 17.Musgrave DS, Bosch P, Lee JY, Pelinkovic D, Ghivizzani SC, Whalen J, et al. Ex vivo gene therapy to produce bone using different cell types. Clin Orthop. 2000;(378):290–305. doi: 10.1097/00003086-200009000-00040. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, et al. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A(7):1032–9. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Wright V, Peng H, Usas A, Young B, Gearhart B, Cummins J, et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6(2):169–78. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 20.Shen HC, Peng H, Usas A, Gearhart B, Cummins J, Fu FH, et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004;34(6):982–92. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Musgrave DS, Pruchnic R, Bosch P, Ziran BH, Whalen J, Huard J. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br. 2002;84(1):120–7. doi: 10.1302/0301-620x.84b1.11708. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D, et al. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13(10):1201–11. doi: 10.1089/104303402320138989. [DOI] [PubMed] [Google Scholar]
- 23.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–27. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Usas A, Gearhart B, Young B, Olshanski A, Huard J. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9(6):885–94. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Peng H, Usas A, Hannallah D, Olshanski A, Cooper GM, Huard J. Noggin improves bone healing elicited by muscle stem cells expressing inducible BMP4. Mol Ther. 2005;12(2):239–46. doi: 10.1016/j.ymthe.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Lee CW, Fukushima K, Usas A, Martinek V, Pelinkovic D, Musgrave D, et al. Myoblast mediated gene therapy with muscle as a biological scaffold for the repair of full-thickness defects of articular cartilage. Transactions of the 46th Annual Meeting, Orthopaedic Research Society; 2000; Orlando, Florida. 2000. [Google Scholar]
- 28.Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, et al. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29(9):1920–30. [PubMed] [Google Scholar]
- 29.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433–42. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 30.Cox S, Cole M, Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10(5–6):942–54. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 31.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12(6):1587–95. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 32.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12(8):2385–96. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 33.Frisk T, Rydholm S, Andersson H, Stemme G, Brismar H. A concept for miniaturized 3-D cell culture using an extracellular matrix gel. Electrophoresis. 2005;26(24):4751–8. doi: 10.1002/elps.200500478. [DOI] [PubMed] [Google Scholar]
- 34.Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323–33. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itescu S, Schuster MD, Kocher AA. New directions in strategies using cell therapy for heart disease. J Mol Med. 2003;81(5):288–96. doi: 10.1007/s00109-003-0432-0. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, et al. Heart muscle engineering: an update on cardiac muscle replacement therapy Heart repair and stem cells. Cardiovasc Res. 2006;71(3):419–29. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 37.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. 2006;577(Pt 2):467–78. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, et al. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12(6):1130–41. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 39.Payne TR, Oshima H, Sakai T, Ling Y, Gharaibeh B, Cummins J, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12(16):1264–74. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(1):31–7. doi: 10.1007/s00192-002-1004-5. discussion 7. [DOI] [PubMed] [Google Scholar]
- 41.Huard J, Yokoyama T, Pruchnic R, Qu Z, Li Y, Lee JY, et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002;9(23):1617–26. doi: 10.1038/sj.gt.3301816. [DOI] [PubMed] [Google Scholar]
- 42.Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, et al. Adult Stem Cell Therapy of Female Stress Urinary Incontinence. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. Single institution clinical trial of muscle-derived cell injection to treat stress urinary incontinence. Abstracts of the American Urological Association Annual Meeting; 2006; Atlanta, Georgia. 2006. [Google Scholar]
- 44.Ott HC, Davis BH, Taylor DA. Cell therapy for heart failure--muscle, bone marrow, blood, and cardiac-derived stem cells. Semin Thorac Cardiovasc Surg. 2005;17(4):348–60. doi: 10.1053/j.semtcvs.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96(2):151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 46.Ye L, Haider H, Sim EK. Adult stem cells for cardiac repair: a choice between skeletal myoblasts and bone marrow stem cells. Exp Biol Med (Maywood) 2006;231(1):8–19. doi: 10.1177/153537020623100102. [DOI] [PubMed] [Google Scholar]