Abstract
Autoimmune diseases arise from a break in tolerance toward self‐antigens and are characterised by the development of pathogenic T cell populations infiltrating the target organ. Regulatory T cells play an important role in maintaining self‐tolerance through their inhibitory functions on effector T cells. The usage of ex vivo‐generated regulatory T cells (Treg) has been regarded as a potentially attractive therapeutic approach for autoimmune diseases. However, the dynamics of Treg in autoimmunity are not well understood. Here, we summarise our published findings on the interplay between Treg and Th17 effector cells in vivo during experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. We have developed Foxp3gfp “knock‐in” mice and myelin oligodendrocyte glycoprotein (MOG)35–55/IAb (MHC class II)‐tetramers to track autoantigen‐specific Treg in vivo during EAE. On immunisation with MOG, Treg cells were detected in the central nervous system (CNS) as early as day 10. However, at the onset of disease, the accumulation of MOG‐specific effector T cells (T‐eff) largely prevailed. Subsequently, during remission T‐eff rapidly contracted whereas a highly suppressive Treg population persisted in the CNS. The interplay between effector Th17 and Treg extend beyond their functions in vivo as we and others have identified the factors responsible for Th17 differentiation. While TGF‐β is a critical differentiation factor for Treg cells, IL6 completely inhibits the generation of Treg cells induced by TGF‐β. Instead, IL6 and TGF‐β together induce the differentiation of pathogenic Th17 cells. Our data demonstrate a dichotomy in the generation of Th17 cells that induce autoimmunity and Treg cells that inhibit autoimmune tissue injury.
CD4 CD25+ regulatory T cells (Treg) are critical for the control of autoimmunity and tissue injury.1 These CD4 CD25+ Treg cells are generated in the thymus, presumably by moderately high affinity interaction of developing autoreactive T cell receptor (TCR)‐bearing T cells with self‐antigens presented in the thymus.2 Once generated, the Treg cells are seeded to the peripheral immune compartment where they regulate activation and effector functions of autopathogenic T cells throughout the life of an individual. The existence of CD4 CD25+ Treg cells in humans3 and their role in the regulation of human autoimmune diseases have been suggested as well.4,5 The potential role of this subset of T cells in multiple sclerosis (MS) has recently been analysed. CD4 CD25+ Treg cells are present at a lower frequency in patients with MS and are defective in their suppressor functions in vitro.6 FOXP3 is a transcription factor that is specifically expressed in CD4 CD25+ Treg cells.7 Its expression is crucial for their anergic phenotype in vitro and their suppressor function.8 Recently, we have generated Foxp3gfp “knock‐in (KI)” mice on the C57BL/6 background in which Foxp3 expressing Treg cells can be tracked in vivo by the expression of the green fluorescent protein (GFP). We have also engineered a myelin oligodendrocyte glycoprotein (MOG)35–55/IAb tetramer to identify MOG‐specific effector T cells (T‐eff) and Treg cells in vivo. Combining these two technologies, we have now studied the interaction and the function of MOG35–55‐specific pathogenic and regulatory T cells during experimental autoimmune encephalomyelitis (EAE). We show that MOG35–55/IAb tetramer‐reactive FOXP3/GFP+ T cells exist in vivo and can readily traffic to the central nervous system (CNS) where they rapidly develop suppressive properties. Whereas the number and cytokine phenotype of the FOXP3/GFP+ Treg cells do not dramatically change in the peripheral immune compartment, there is a remarkable change in the frequency and the functional phenotype of the Treg cells in the CNS during experimental autoimmune encephalomyelitis (EAE), which particularly coincides with the clinical recovery from disease.9
Myelin antigen‐specific Treg cells can be expanded in the peripheral immune compartment by immunisation with MOG35–55
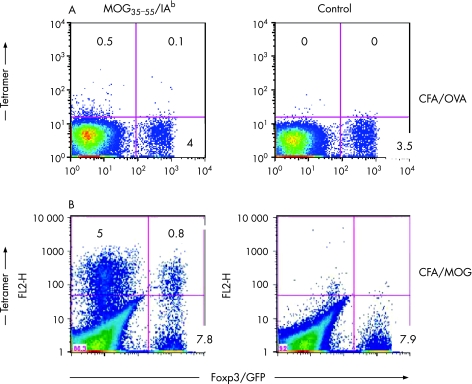
While Treg populations can be detected during the development of an immune response, it is not known whether autoantigen‐specific Treg cells are expanding in vivo following immunisation with encephalitogenic myelin antigens. To address this question, we used Foxp3gfp KI mice along with MOG35–55/IAb tetramers and tracked FOXP3/GFP– T cells (T‐eff) and FOXP3/GFP+ T cells (Treg) based on their myelin antigen specificity. Ex vivo staining of splenocytes with MOG35–55/IAb tetramers 9 days after immunisation revealed a consistent frequency of 5% of tetramer‐reactive CD4 GFP– T‐eff cells and 0.5% of tetramer‐reactive CD4 GFP+ Treg cells (fig 1). The isolation of T cells from the brain of Foxp3 GFP KI mice immunised with MOG35–55 demonstrated that, in contrast to the periphery, there was a big change in the numbers of T‐eff and Treg cells in the CNS of these animals. However, it was not clear what percentage of these cells were antigen‐specific and how this might correlate with the clinical disease course.
Figure 1 Antigen‐specific Treg cells are expanded upon immunisation with MOG35–55 lymph node cells and splenocytes were isolated from unimmunised Foxp3gfp.KI‐mice or from Foxp3gfp.KI‐mice that had been sensitised in vivo with MOG35–55/CFA or OVA323–339/CFA 8 days before. The cells were stained with MOG35–55/IAb tetramers. Live (7AAD–) CD4 T cell populations are shown. The numbers in each quadrant represent percentages.
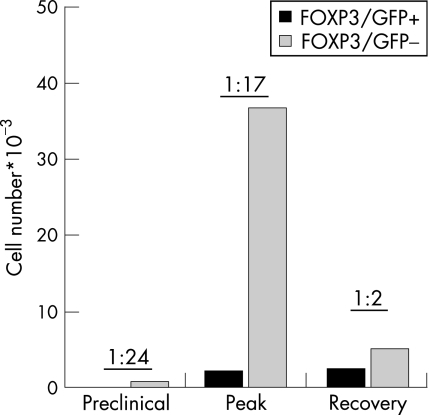
To answer this question, MOG35–55/IAb tetramer staining was performed on CNS‐derived mononuclear cells. Consistent with the current pathogenic concept of MOG‐induced EAE, the frequency of MOG35–55‐specific T‐eff cells (MOG35–55/IAb tetramer+FOXP3–) was very high at the disease peak and dropped dramatically during recovery (fig 2). Similarly to the frequency observed in the periphery, 0.5% of all CD4 cells were MOG35–55‐specific Treg cells (MOG35–55/IAb tetramer+FOXP3+) at the peak of the disease. However, as the clinical signs improved during recovery, the frequency of MOG35–55‐specific Treg cells increased to close to 1% of all CNS‐derived CD4 cells. The decreased frequency of effector T cells relative to regulatory T cells was reflected by the absolute numbers of each population and there was a dramatic drop in the number of MOG35–55/IAb tetramer+ T‐eff cells when the animals proceeded from maximum disease to recovery (fig 2). In contrast, the number of the myelin‐specific Treg cells in the CNS remained constant or even slightly increased during the transition from peak to clinical recovery. Indeed, the ratio of MOG35–55‐specific Treg and T‐eff cells in the CNS was 1:24 and 1:17 at the onset and peak of EAE, but changed to 1:2 at the beginning of recovery. No other lymphoid tissue (including the peripheral blood) showed similar high frequencies of myelin‐specific Treg cells. Thus, myelin‐specific Treg cells target the CNS and accumulate in the CNS where they constitute a stable pool of CNS‐residing regulatory cells. Therefore, there is a particularly accurate correlation between the clinical course of EAE and the ratio of MOG35–55‐specific Treg and T‐eff cells from the CNS, but not from the draining lymph nodes. Furthermore, when analysing the dynamics of cytokine production during the course of disease, we observed that Treg cells from the CNS produced large quantities of IL10 immediately preceding the clinical recovery, a phenomenon that was not observed in any lymphoid compartment but the CNS. Thus, the ratio between T‐eff and Treg cells within the CNS infiltrates and the presence of IL10‐producing Treg cells in CNS was closely correlated with the initiation of clinical remission. The dynamics and phenotype of Treg cells in the peripheral lymphoid compartment including the draining lymph nodes strikingly lacked this clear correlation.
Figure 2 Myelin‐specific Treg cells accumulate in the CNS. Mononuclear cells were prepared from the CNS of MOG/CFA‐immunised mice at different stages of EAE as indicated. Absolute numbers of MOG‐tetramer+ Treg vs T‐eff recovered from the CNS before disease onset (preclinical), at the peak of disease and during recovery. The ratio of MOG‐specific Treg:T‐eff is indicated for each disease phase.
As soon as tolerance is broken, massive expansion of autoreactive T cells and evasion into the target tissue occur. We illustrate that myelin‐specific Treg cells, very much like T‐eff cells, migrate to the target organ with only negligible delay. It is very likely that engagement of their myelin‐specific TCR relocates Treg cells to the CNS and is also essential for their re‐activation in situ. There is evidence that natural Treg cells might not perform bystander suppression, but compete with T‐eff cells for the same autoantigen.10,11
Role of Th17 cells in autoimmunity
Th17 cells represent a new subset of T helper cells, which mainly produce IL17A and IL17F, IL22 and, to a lesser extent, TNFα. The first report on IL17‐producing CD4 T cells came from a study of in vitro‐primed TcR transgenic T cells where the addition of Borrelia burgdorferi lysate induced IL17 production.12 However, in the last 3 years the outstanding importance of Th17 cells has most convincingly been demonstrated in the pathogenesis of organ‐specific autoimmune diseases. This was paradigm changing, since previously Th1 cells had been regarded as the pathogenic cells driving autoimmune tissue damage.13 This concept was challenged when it became clear that IFNγ and IFNγ‐receptor deficient mice, as well as mice that lack other molecules involved in the differentiation and stabilisation of the Th1 phenotype like IL12p35, IL12 receptor‐β2 and IL18, were not protected from EAE, but developed more severe disease.14,15,16,17,18 Furthermore, it was shown that IL23 and not IL12 was crucial for mounting an autopathogenic T cell response in the CNS,19 suggesting that Th1 might be dispensable for the development of organ‐specific autoimmune diseases. Finally, the observations that Th17 cells were more potent than Th1 cells in transferring EAE to naive wild‐type host animals20 suggested that Th17 cells might be responsible for the induction of tissue‐specific autoimmunity. IL17 is directly involved in cartilage and bone destruction as observed in an experimental model for human rheumatoid arthritis.21 Similarly, IL17‐deficient animals develop experimental autoimmune encephalomyelitis (EAE) with delayed onset and diminished severity.22 In addition, administration of an IL17‐blocking antibody in mice immunised with a myelin antigen prevents chemokine expression in the brain and the subsequent development of EAE.20,23 Collectively, these data corroborate the importance of Th17 cells for the induction of autoimmune tissue inflammation.
The differentiation of Th17 cells
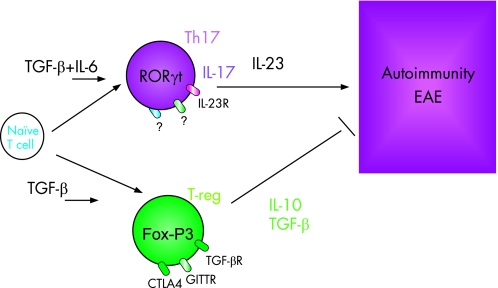
While a clear role of IL23 and Th17/IL17 had been established, the factors that were necessary for their development from naive T cells had remained elusive. While early studies had suggested that IL23 could drive their differentiation, it quickly became clear that this cytokine was important for the Th17 subset but did not act on naive T cells. Three independent studies independently observed that a combination of the pro‐inflammatory cytokine IL6 and TGF‐β could induce the differentiation of Th17 cells from naive T cells in vitro.24,25,26 The importance of TGF‐β and IL6 for this process in vivo has been shown as well. We have demonstrated that mice, which express TGF‐β under the CD2 promoter, produce TGF‐β on stimulation ex vivo, but developed Th17 cells in vivo under inflammatory conditions and elevated concentrations of IL6. The increased number of Th17 cells in these animals resulted in the exacerbation of EAE. The analysis of IL6‐deficient animals also points to an important role of IL6 in the differentiation of Th17 cells since these animals are resistant to the development of EAE and do not develop a competent Th17 cell response.25 Therefore, under inflammatory conditions and constant surveillance by regulatory mechanisms, IL6 and TGF‐β appear to be key cytokines for the differentiation of Th17 cells in vivo (fig 3).
Figure 3 Reciprocal developmental pathways for the generation of pathogenic effector T Th17 cells and regulatory T cells. At the steady‐state level TGF‐β produced in the immune system will suppress the generation of effector T cells and induce Foxp3+ regulatory T cells, and thereby maintain self‐tolerance. However, on infection or inflammation, IL6 produced by the activated innate immune system suppresses the generation of TGF‐β‐induced Treg cells and induces pro‐inflammatory Th17 cells.
Taken together, there is a functional antagonism between Th17 and Treg cells, and there is a dichotomy in their generation as well. Treg cells and Th17 effectors arise in a mutually exclusive fashion, depending on whether they are activated in the presence of TGF‐β or TGF‐β plus IL6. At the steady‐state level or in the absence of any inflammatory insult, TGF‐β produced in the immune system will suppress the generation of effector T cells and induce Foxp3+ regulatory T cells, and thereby maintain self‐tolerance. However, on infection or inflammation, IL6 produced by the activated innate immune system suppresses the generation of TGF‐β−induced Treg cells and induces a pro‐inflammatory T‐cell response predominated by Th17 cells (fig 3). Although IL6 plays a critical role in the development of the Th17 response and the inhibition of Treg functions, we also believe that additional control steps must exist during this process and that other cytokines might participate in Th17 differentiation.25,27
In the natural course of MOG35–55‐induced EAE, the number of Th17 cells producing CD4 T cells in the CNS peaks earlier than Th1 cells and it is known that many chemokine genes are targets of IL17.9 In the current understanding, antigen‐specific priming of encephalitogenic T cells as well as their commitment to a certain T helper cell lineage happens in secondary lymphoid tissue outside the CNS. Myelin‐specific T cells then traffic to the CNS where they are re‐activated.28,29 The efficiency of the activation in situ, and maintenance within the target tissue as well as the acquisition of further effector functions is governed by recognition of the cognate antigen within the CNS. Thus, a selected population of effector T cells is accumulating in the CNS. Based on this hypothesis, Th17 cells may infiltrate the target tissue first and prepare the ground for the infiltration of Th1 cells. However, in order to drive severe tissue inflammation, the Th17 cells response must not be too short‐lived. Indeed, when IL23 is not available in order to maintain and expand a population of already primed Th17 cells, EAE is severely attenuated or even abrogated.19 This suggests that IL23p19 is required to shape a stable Th17 cell population in the secondary lymphoid tissue, and also to maintain – at least temporarily – an encephalitogenic Th17 population in the CNS.
Collectively, Th17 cells are highly potent inflammatory cells that initiate tissue inflammation and induce the infiltration of other inflammatory cells into the target organ. Hence, at least in relevant mouse models of organ‐specific autoimmunity, Th17 cells are indispensable for the induction of massive immunopathology. Paradoxically and unlike Th1 cells, Th17 cells may not be subject to Treg‐mediated suppression. However, it appears that in order for Th17 cell populations to be maintained in the target tissue a complex network of cytokines has to be operative which provides the necessary means to control Th17 cells and may be the basis for the fact that this T cell subset is relatively short‐lived and prone to rapid attrition.
Abbreviations
EAE - experimental autoimmune encephalomyelitis
MOG - myelin oligodendrocyte glycoprotein
MS - multiple sclerosis
T‐eff - effector T cells
Treg - regulatory T cells
Footnotes
Competing interests: None declared.
References
- 1.Sakaguchi S. Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nat Immunol 20056345. [DOI] [PubMed] [Google Scholar]
- 2.Jordan M S, Boesteanu A, Reed A J, Petrone A L, Holenbeck A E, Lerman M A, Naji A, Caton A J. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self‐peptide. Nat Immunol 20012301. [DOI] [PubMed] [Google Scholar]
- 3.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk A H. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 20011931285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baecher‐Allan C, Hafler D A. Suppressor T cells in human diseases. J Exp Med 2004200273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenstein M R, Evans J G, Singh A, Moore S, Warnes G, Isenberg D A, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti‐TNFalpha therapy. J Exp Med 2004200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas J, Hug A, Viehover A, Fritzsching B, Falk C S, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, Storch‐Hagenlocher B, Krammer P H, Suri‐Payer E, Wildemann B. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol 2005353343. [DOI] [PubMed] [Google Scholar]
- 7.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity 200319165. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot J D, Rasmussen J P, Williams L M, Dooley J L, Farr A G, Rudensky A Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 200522329. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen T R, Backstrom B T, Sobel R A, Wucherpfennig K W, Strom T B, Oukka M, Kuchroo V K. Myelin‐specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 200713423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein L, Trautman L, Psarras S, Schnell S, Siermann A, Liblau R, von Boehmer H, Khazaie K. Visualizing the course of antigen‐specific CD8 and CD4 T cell responses to a growing tumor. Eur J Immunol 200333806. [DOI] [PubMed] [Google Scholar]
- 11.Tarbell K V, Yamazaki S, Olson K, Toy P, Steinman R M. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 20041991467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infante‐Duarte C, Horton H F, Byrne M C, Kamradt T. Microbial lipopeptides induce the production of IL‐17 in Th cells. J Immunol 20001656107. [DOI] [PubMed] [Google Scholar]
- 13.O'Garra A, Steinman L, Gijbels K. CD4+ T‐cell subsets in autoimmunity. Curr Opin Immunol 19979872. [DOI] [PubMed] [Google Scholar]
- 14.Krakowski M, Owens T. Interferon‐gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol 1996261641. [DOI] [PubMed] [Google Scholar]
- 15.Tran E H, Prince E N, Owens T. IFN‐gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol 20001642759. [DOI] [PubMed] [Google Scholar]
- 16.Gran B, Zhang G X, Yu S, Li J, Chen X H, Ventura E S, Kamoun M, Rostami A. IL‐12p35‐deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL‐12 system in the induction of central nervous system autoimmune demyelination. J Immunol 20021697104. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G X, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL‐12 receptor‐beta 2‐deficient mice: IL‐12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 20031702153. [DOI] [PubMed] [Google Scholar]
- 18.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18‐independent engagement of interleukin 18 receptor‐alpha is required for autoimmune inflammation. Nat Immunol 20067946. [DOI] [PubMed] [Google Scholar]
- 19.Cua D J, Sherlock J, Chen Y, Murphy C A, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira S A, Gorman D, Kastelein R A, Sedgwick J D. Interleukin‐23 rather than interleukin‐12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003421744. [DOI] [PubMed] [Google Scholar]
- 20.Langrish C L, Chen Y, Blumenschein W M, Mattson J, Basham B, Sedgwick J D, McClanahan T, Kastelein R A, Cua D J. IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005201233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua D J, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 20062032673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL‐17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006177566. [DOI] [PubMed] [Google Scholar]
- 23.Hofstetter H H, Karulin A Y, Forsthuber T G, Ott P A, Tary‐Lehmann M, Lehmann P V. The cytokine signature of MOG‐specific CD4 cells in the EAE of C57BL/6 mice. J Neuroimmunol 2005170105. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, Hocking R J, Atkins C J, Locksley R M, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL‐17‐producing T cells. Immunity 200624179. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Korn T, Strom T B, Oukka M, Weiner H L, Kuchroo V K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006441235. [DOI] [PubMed] [Google Scholar]
- 26.Mangan P R, Harrington L E, O'Quinn D B, Helms W S, Bullard D C, Elson C O, Hatton R D, Wahl S M, Schoeb T R, Weaver C T. Transforming growth factor‐beta induces development of the T(H)17 lineage. Nature 2006441231. [DOI] [PubMed] [Google Scholar]
- 27.Nardelli D T, Cloute J P, Luk K H, Torrealba J, Warner T F, Callister S M, Schell R F. CD4(+) CD25(+) T cells prevent arthritis associated with Borrelia vaccination and infection. Clin Diagn Lab Immunol 200512786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wekerle H, Kojima K, Lannes‐Vieira J, Lassmann H, Linington C. Animal models. Ann Neurol 199436(Suppl)S47. [DOI] [PubMed] [Google Scholar]
- 29.Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne D E, Li Z, Ellwart J W, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity 200114547. [DOI] [PubMed] [Google Scholar]