Abstract
Sepsis is a severe complication of abdominal infections such as peritonitis and is associated with high mortality. Unfortunately, the molecular mechanisms controlling the development of sepsis are still incompletely understood. Interestingly, the interleukin (IL) 12 family member IL27 seems to play a key role in sepsis. In a murine model of septic peritonitis induced by caecal ligation and puncture (CLP), IL27 levels were found to be strongly induced. Furthermore, mice deficient for the EBI3 subunit of IL27 were resistant to CLP‐induced septic peritonitis as compared to wild‐type controls. This effect could be suppressed by injection of recombinant IL27. Further studies demonstrated that IL27 directly suppresses endotoxin‐induced production of reactive oxygen intermediates by isolated primary murine granulocytes. The significance of this observation was underlined by studies on in vivo blockade of IL27 function using a newly designed soluble IL27 receptor fusion protein (sWSX‐Fc). Such treatment led to significantly increased survival after CLP as compared to control‐treated mice. These findings indicate that IL27 is an important negative regulator of innate immune cells in septic peritonitis. Blockade of IL27 function could be an interesting novel approach for the treatment of patients with sepsis.
The gut is the largest immune organ of the human body and contains numerous antigen presenting cells and T lymphocytes. An important mechanism in the efferent arm of the intestinal immune system consists of the activation and differentiation of T lymphocytes, in particular CD4 and CD8 lymphocytes. The differentiation of CD4 T lymphocytes in functionally distinct subpopulations represents a key mechanism of the mucosal immune defence against pathogens, and also for the development of tolerance against mucosal antigens.
Several subtypes of CD4 T helper (Th) cells can be distinguished based on their cytokine profile. Th1 cells are characterised by the production of the pro‐inflammatory cytokines interferon γ and tumour necrosis factor, whereas Th2 cells secret IL4, IL5, IL6, IL9 and IL13, which are pro‐inflammatory. Recent data suggest that further distinct T helper cell populations can be distinguished. Th3 cells produce the anti‐inflammatory TGF‐β, regulatory CD25 CD4 (Tr) cells produce large amounts of the anti‐inflammatory IL10. Finally, Th17 cells express the master transcription RORgammat and produce IL17 and IL22. These cells are induced from naive T cells via IL6 and TGF‐β and seem to require IL23 for stabilisation of their phenotype.
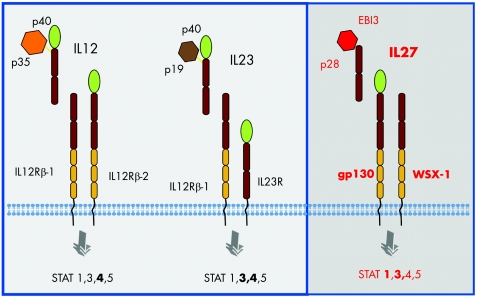
IL27 was identified in 2002 by Pflanz and coworkers as a new bioactive member of the IL12 cytokine family.1 IL27 has two different subunits: an IL12 p40‐related polypeptide, denoted EBV‐induced gene 3 (EBI3) and a novel p28 subunit (fig 1).1,2 It mediates its biological function via binding to a specific receptor on target cells consisting of the orphan receptor WSX‐1/TCCR and the widely expressed gp130 protein.3 Over the past few years, IL27 has emerged as a pivotal cytokine in the adaptive immune system by controlling T cell‐dependent immune responses. Specifically, IL27 activates STAT1 and STAT3 in naive CD4 T cells and natural killer (NK) cells. While STAT1 phosphorylation is required for IL27‐mediated activation of the Th1 master transcription factor T‐bet, STAT3 is considered to be important for IL27‐induced T cell proliferation.4
Figure 1 IL12 family members. The structure and signalling of IL12 family members is shown. IL27 consists of a p28 and an EBI3 subunit that signal via gp130 and WSX‐1. The structures of IL27 and IL23 are also shown.
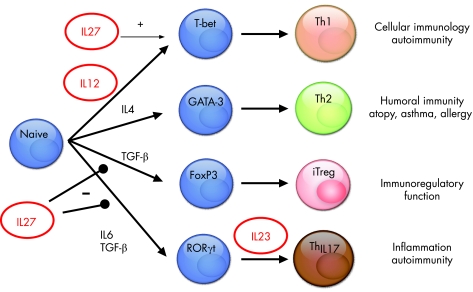
Antigen presenting cells such as dendritic cells and macrophages have been identified as rapid producers of IL27 subunits after toll‐like receptor (TLR) ligation .5 In fact, lipopolysaccharide (LPS) stimulated dendritic cells showed expression of both p28 and EBI3 before expression of IL12 p35/p40 subunits, suggesting that IL27 may act early in Th1‐mediated immunity (fig 2). However, recent studies demonstrated that the biological function of IL27/WSX‐1 signalling is more complex, as it is also critically involved in the negative control of both Th1 and Th2 inflammatory responses.6,7,8 Finally, mice deficient for the EBI3 subunit of IL27 showed reduced invariant NK T cell numbers and cytokine production in colitis, suggesting that EBI3 controls invariant NKT (iNKT) cell activity.9 Finally, IL27 signalling has also been implicated in STAT3‐dependent, negative regulation of murine mast cells and activated macrophages.6 In the next paragraphs, the role of IL27 in sepsis will be discussed.10
Figure 2 Role of IL27 family members in T cell differentiation. IL27 seems to favour Th1 differentiation, while it suppresses iTreg and Th17 differentiation. In contrast, IL23 activates Th17 cells and IL12 induces early Th1 differentiation.
Sepsis is a clinical syndrome with severe infection in the body and bloodstream.11,12 Although sepsis is generally associated with a significant mortality, patients with septic peritonitis have a particularly high mortality rate of up to 80%. Septic peritonitis is characterised by a massive infiltration of neutrophils and macrophages into the peritoneal cavity where these cells are the first line of defence for attacking invading micro‐organisms. However, once such cells fail to restrict microbes to the peritoneal cavity, microbes may reach the bloodstream, resulting in an overwhelming systemic immune response via the production of pro‐inflammatory and anti‐inflammatory mediators. Such mediators in turn may cause shock or multi‐organ failure, thereby explaining the high mortality associated with sepsis.12
In a recent study, Wirtz and coworkers10 analysed the expression of IL27 subunits in the murine CLP model of bacterial peritonitis. It was found that EBI3 and p28 mRNA levels in the lung and spleen were substantially increased 6 hours after CLP. Furthermore, a strong upregulation of IL27 protein in blood and peritoneal lavage fluid was observed shortly after CLP. In addition, experiments with isolated resident peritoneal macrophages and neutrophils demonstrated that these cells produce large amounts of IL27 on stimulation with LPS.
To analyse the functional role of IL27, mice deficient in the EBI3 subunit of IL27 were studied. Interestingly, CLP survival rates in EBI3−/− mice were significantly higher than in the wild‐type control group. While all wild‐type mice died within 4 days after CLP, approximately 50% of the EBI3−/− mice survived for more than 2 weeks. In addition, intraperitoneal (ip) injection of 4x108 live E coli led to high lethality in wild‐type mice, whereas all EBI3−/− mice survived, suggesting that IL27 EBI−/− mice are resistant to the lethality induced by microbial infections. Finally, Wirtz and coworkers observed that treatment with rIL27 markedly increased the mortality of IL27 EBI3 deficient mice, suggesting that IL27 exacerbates septic peritonitis.
In functional studies, Wirtz and coworkers10 next compared the bactericidal activity of granulocytes and monocytes between IL27 EBI3−/− and IL27 EBI3+/+ mice by analysing reactive oxygen intermediates (ROI). Mice were injected with 1.5 ml 3% thioglycolate for 4 h and 24 h to generate an inflammatory stimulus and their potency to produce ROI was determined using flow cytometry. Interestingly, granulocytes from EBI3−/− mice displayed a markedly enhanced ability to produce ROI as compared to EBI3+/+ mice, suggesting that IL27 negatively regulates the biological functions of granulocytes.
Since EBI3−/− mice were protected from septic peritonitis, Wirtz and coworkers10 finally determined the potential beneficial effects of neutralising the biological function of IL27 in vivo. A fusion protein of the extracellular part of WSX‐1 with the Fc part of IgG was used to block IL27 function in vivo. Importantly, the survival rates in sWSX‐Fc treated mice were significantly higher than in the saline‐treated control group, showing that blockade of IL27 is an effective therapy for experimental septic peritonitis.
A better understanding of the pathophysiology of sepsis is required to develop rational novel strategies for sepsis.11,12 In particular, the regulation of neutrophil functions during septic peritonitis is still incompletely understood. Wirtz and coworkers10 found that IL27 subunits were rapidly produced by both macrophages and neutrophils during septic peritonitis. This rapid production is presumably due to augmented EBI3 and p28 gene transcription as both promoters contain TLR‐responsive NFκB binding elements. These data suggest a novel key role of IL27 in regulating innate immunity and neutrophil function during septic peritonitis. Taken together, the available data suggest a major role of IL27 in controlling oxidative bursts of granulocytes and elimination of bacteria during peritonitis. The therapeutic implication of these observations was shown by blockade of the IL27 function using a Wsx‐Fc fusion protein in vivo. Such blockade of the IL27 function by WSX‐Fc emerges as a novel treatment modality for patients with septic peritonitis. It will be interesting to see whether patients with sepsis will benefit from such anti‐IL27 strategies in the future.
Abbreviations
CLP - caecal ligation and puncture
IL - interleukin
LPS - lipopolysaccharide
NK - natural killer
ROI - reactive oxygen intermediates
Th cells - T helper cells
TLR - toll‐like receptor
Footnotes
Competing interests: None declared.
References
- 1.Pflanz S, Timans J C, Cheung J, Rosales R, Kanzler H, Gilbert J.et al IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 200216779–790. [DOI] [PubMed] [Google Scholar]
- 2.Devergne O, Hummel M, Koeppen H, Le Beau M M, Nathanson E C, Kieff E.et al A novel interleukin‐12 p40‐related protein induced by latent Epstein‐Barr virus infection in B lymphocytes. J Virol 1996701143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan J F.et al WSX‐1 and glycoprotein 130 constitute a signal‐transducing receptor for IL‐27. J Immunol 20041722225–2231. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL‐27‐induced T‐bet expression but not proliferation of naive CD4+ T cells. J Immunol 20041733871–3877. [DOI] [PubMed] [Google Scholar]
- 5.Wirtz S, Becker C, Fantini M C, Nieuwenhuis E E, Tubbe I, Galle P R.et al EBV‐induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF‐kappa B activation. J Immunol 20051742814–2824. [DOI] [PubMed] [Google Scholar]
- 6.Artis D, Villarino A, Silverman M, He W, Thornton E M, Mu S.et al The IL‐27 receptor (WSX‐1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol 20041735626–5634. [DOI] [PubMed] [Google Scholar]
- 7.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H.et al The IL‐27R (WSX‐1) is required to suppress T cell hyperactivity during infection. Immunity 200319645–655. [DOI] [PubMed] [Google Scholar]
- 8.Villarino A V, Stumhofer J S, Saris C J, Kastelein R A, de Sauvage F J, Hunter C A. IL‐27 limits IL‐2 production during Th1 differentiation. J Immunol 2006176237–247. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwenhuis E E, Neurath M F, Corazza N, Iijima H, Trgovcich J, Wirtz S.et al Disruption of T helper 2‐immune responses in Epstein‐Barr virus‐induced gene 3‐deficient mice. Proc Natl Acad Sci USA 20029916951–16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirtz S, Tubbe I, Galle P R, Schild H J, Birkenbach M, Blumberg R S.et al Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med 20062031875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hepburn M J, Purcell B K, Paragas J. Pathogenesis and sepsis caused by organisms potentially utilized as biologic weapons: opportunities for targeted intervention. Curr Drug Targets. 2007, in press [DOI] [PubMed]
- 12.Leaver S K, Finney S J, Burke‐Gaffney A, Evans T W. Sepsis since the discovery of Toll‐like receptors: disease concepts and therapeutic opportunities. Crit Care Med. 2007, in press [DOI] [PubMed]