Abstract
No new therapy has been approved for systemic lupus erythematosus (SLE) in decades. Interest in SLE by pharmaceutical and biotechnology companies has increased, leading to multiple clinical trials. Unfortunately, we have now compiled quite a long list of “failed” trials. If this was due to the fact that the studied therapy did not work in SLE, we could accept it and move on. Of concern, however, is that many of the “failed” treatments had a strong “signal” of efficacy, often in subgroup analyses that made logical sense, given what was known about the mechanism of action of the treatment. This has led, understandably, to concern that there is something wrong with SLE trial designs, particularly with SLE disease activity indices.
The question is, are current disease activity indices any good? There may be more lupus activity indices than systemic lupus erythematosus (SLE) clinical trials. Some are summarised in table 1. Each has strengths and weaknesses; none is perfect. Most are global disease activity indices, giving a summary score of the entirety of SLE activity. One, the British Isles Lupus Activity Group (BILAG) index, is organ‐specific, although it can be converted into a global score.1
Table 1 Characteristics of the commonly used SLE disease activity indices.
| BILAG | SLEDAI | SLAM‐R | ECLAM | LAI | |
|---|---|---|---|---|---|
| Score | A–E | 0–105 | 0–81 | 0–10 (max 17.5) | 0–3 |
| Fatigue items | Yes | No | Yes | Yes | Yes |
| Weighted | No | Yes | Yes | Yes | Yes |
| Laboratory variables | Yes | Yes | Yes | Yes | Yes |
| Complement levels | No | Yes | No | Yes | Yes |
| Anti‐dsDNA | No | Yes | No | No | Yes |
| VAS | No | No | No | No | Yes (scored) |
| Time frame considered | 1 month | Last 10 days | 1 month | Last 1–3 months | 2 weeks |
Adapted from Strand et al.2
Two of the indices, the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and the BILAG, have been the predominant ones used in randomised clinical trials. The BILAG requires the physician to score organ manifestations as improved (1), same (2), worse (3) or new (4) since the last month. Within each organ system, multiple manifestations and/or laboratory tests need to be combined into a single score for that organ. This is accomplished by a computer software program, BLIPS. The organ scores generated are A (“active”), B (“beware”), and C (“contentment”) when there is activity, and D (“resolved activity”) or E (“never involved”) when there is not.
The SLEDAI consists of a list of organ manifestations, each with a definition. The physician decides whether each manifestation is “present” or “absent” in the last 10 days. It is a weighted instrument, in which descriptors are multiplied by that organ's “weight”. For example, arthritis is multiplied by 4, renal descriptors by 4, and central nervous system descriptors by 8. These weighted organ manifestations are then totalled into the final score.
Lupus activity indices have proven validity, when the gold standard is the physician assessment (table 2). To be useful in clinical trials, they must also be able to demonstrate change over time. This, too, has been demonstrated for multiple indices,3 regardless of whether the sensitivity to change is measured in terms of improvement or worsening in disease activity.4
Table 2 Changes in disease activity indices correlate with changes in the physician estimate of activity.
| Disease activity indices | Correlation |
|---|---|
| SLAM | r = 0.54 |
| SLEDAI | r = 0.52 |
| LAI | r = 0.75 |
| BILAG | r = 0.61 |
| ECLAM | r = 0.65 |
Adapted from Ward et al.3
The patient component of disease assessment in SLE is not straightforward. Patients tend to assess fatigue and pain, but in SLE these more likely represent secondary fibromyalgia than immune‐mediated activity of SLE. Some activity indices include patient‐derived assessments, including BILAG and the Systemic Lupus Activity Measure (SLAM). Consequently, these indices are sensitive to change assessed by the patient.3,4 The SLEDAI, however, is not. The Systemic Lupus International Collaborating Clinics (SLICC) has recommended that patient assessment be measured by the SF‐36 and be included under the domain of quality of life, separate from disease activity and organ damage.
Cohort approaches to the definition of flare
Lupus activity encompasses overall disease activity, and also the concept that SLE has “flared”: an increase in activity over a defined amount of time. This concept of flare can, and has, been defined using the existing disease activity indices. Using as a gold standard a 1.0 increase on a 0 to 3 visual analogue scale, “flare” corresponded to a change in 3 points or more on SLAM, 3 points or more on the SLEDAI, or 4 points or more on a global BILAG.5 A separate study of the SLEDAI reached the same conclusion: flare represented a 3 point or more increase, and improvement, a 2 point or more decrement.6 However, the Safety of Estrogen in Lupus: National Assessment (SELENA) investigations did not think these definitions of flare were sufficient, and devised new definitions to separate “mild/moderate” flare from “severe” flare (box 1). These definitions do include SLEDAI cut‐offs, but emphasise treatment. For example, for severe flare, high‐dose prednisone, with addition of an immunosuppressive drug or hospitalisation were included. In fact, these “action” items turned out to identify many more severe flares than the SLEDAI cut‐off. Ultimately, for flare, actions speak louder than activity indices.
Box 1 SELENA definition of flare7
Mild or moderate flare
Change in SELENA‐SLEDAI instrument score of 3 points or more (but not to more than 12)
-
New/worse:
-
-
Discoid, photosensitive, profundus, cutaneous vasculitis, bullous lupus
-
-
Nasopharyngeal ulcers
-
-
Pleuritis
-
-
Pericarditis
-
-
Arthritis
-
-
Fever (SLE)
-
-
Increase in prednisone, but not to >0.5 mg/kg/day
Added NSAID or hydroxychloroquine for SLE activity
⩾1.0 increase in PGA score, but not to more than 2.5
Severe flare
Change in SELENA‐SLEDAI instrument score to greater than 12 points
-
New/worse:
-
-
CNS‐SLE
-
-
Vasculitis
-
-
Nephritis
-
-
Myositis
-
-
Platelet <60 000
-
-
Haemolytic anemia; Hb<70 g/l or decrease in Hb>30 g/l
-
-
Requiring: double prednisone, or prednisone increase to >0.5 mg/kg/day, or hospitalisation
-
-
Increase in prednisone to >0.5 mg/kg/day
New cyclophosphamide, azathioprine, methotrexate for SLE activity
Hospitalisation for SLE
Increase in PGA score to greater than 2.5
Clinically meaningful change
If a treatment for SLE worked, what change in a global disease activity index would occur? For cytotoxic therapy, a mean SLEDAI reduction of 2.59 has been found.8 For leflunomide, a mean SLEDAI reduction of 2.1 was reported.9 These reductions seem very small. One must remember, though, that the only way to lose points on the SLEDAI is if the organ manifestation disappears.
Data‐driven approaches to organ‐specific outcomes
Current SLE disease activity indices were not derived for clinical trials, but for cohort or cross‐sectional studies. In order for them to be used reliably in clinical trials, investigators, not all of whom have used them extensively in clinical research, require intensive training (and testing). Ongoing monitoring of data collection forms is mandatory to ensure that indices are being completed in a uniform way. The translation of disease activity indices from clinical research to clinical trials has been a painful growing process.
It seems inviting to just study one organ in SLE, especially lupus nephritis in which the assessments are made by laboratory tests, avoiding activity indices altogether. The SLICC group tested the agreement of physician rating of renal activity and found a respectable, but not perfect, intraclass correlation coefficient of 0.56. Using real patient scenarios, the issues preventing perfect agreement were that laboratory tests did not all change in the same direction, and that it was often impossible to know if a reduced measure of glomerular filtration rate (GFR) represented “activity” or permanent organ damage. Thus, although the laboratory tests seem “objective”, putting together proteinuria, urine sediment and GFR into one rating is anything but! The SLICC group used regression analysis to build a model for renal activity. In this model, proteinuria terms were the most important.
Successful investigator‐initiated clinical trials in lupus nephritis have not used lupus activity indices. Two induction trials of mycophenolate mofetil versus cyclophosphamide for induction therapy defined complete response for each renal laboratory test.10,11 A clinical trial of mycophenolate mofetil, azathioprine or cyclophosphamide for the maintenance of response in lupus nephritis used renal failure or increase in creatinine as the outcome.12 The SLICC results would suggest that proteinuria might function well as a sole laboratory outcome.
Choice of outcome
Lupus activity indices are not the only issue affecting the success of SLE trial design. The FDA draft guidance statement on SLE clinical trials offered two trial outcomes: prolongation of time to flare or reduction in disease activity. This has led some trials to try to achieve both in a single trial. It has also led to other trials picking one outcome over the other, potentially contributing to a “failed” trial.
For example, given a potential treatment whose mechanism of action suggests benefit for SLE renal disease, what outcome should be chosen? A trial to prolong the time to renal flare will be lengthy; the comparators azathioprine and mycophenolate mofetil work (in other words, there isn't a real placebo group); patients will drop out; lupus treatments will change, leading to protocol deviations, violations and drop‐outs. Or, one could choose to enrol patients with stable proteinuria and determine, perhaps over a time period as short as 3 months, whether the new treatment significantly reduces proteinuria. Ultimately, much can be said for picking a simple, straightforward outcome, if one is available.
Choice of study design
Multiple potential study designs have been used in SLE. “Superiority” designs require that the treatment be better than an existing standard, such as the NIH trials of cyclophosphamide versus corticosteroids for lupus nephritis. “Non‐inferiority” designs ask if a therapy is equivalent to another, within a predefined range. This type of design may require a larger number of patients. It was successfully used to show that oral contraceptive pills do not increase SLE flares in the SELENA study.7
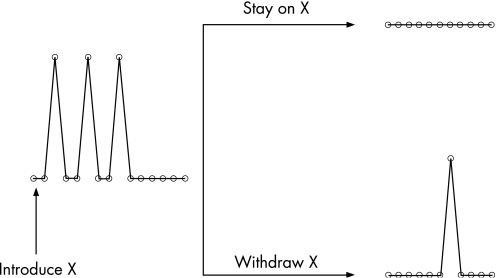
Current SLE randomised clinical trials often require 200–300 patients in Phase 2, with sample sizes of 600 for Phase 3. Given strict inclusion and exclusion criteria, where are we going to find the patients for these trials? One suggestion is to move to simple study designs. One such study design, the randomised withdrawal design, is shown in fig 1. This study design can be used to show that a new treatment reduces flares. Patients with lupus flares are enrolled and started on the new treatment X. We may not know how long it takes for X to work. When the SLE flares have resolved, whether this be in 1, 3 or 6 months, the patient is then eligible for randomisation to continue on X versus placebo. If X works, the placebo arm should have more flares. This trial design was used successfully to show that hydroxychloroquine reduces SLE flares. It required a sample size of only 46.13
Figure 1 Randomised withdrawal study design.
Selection of patients
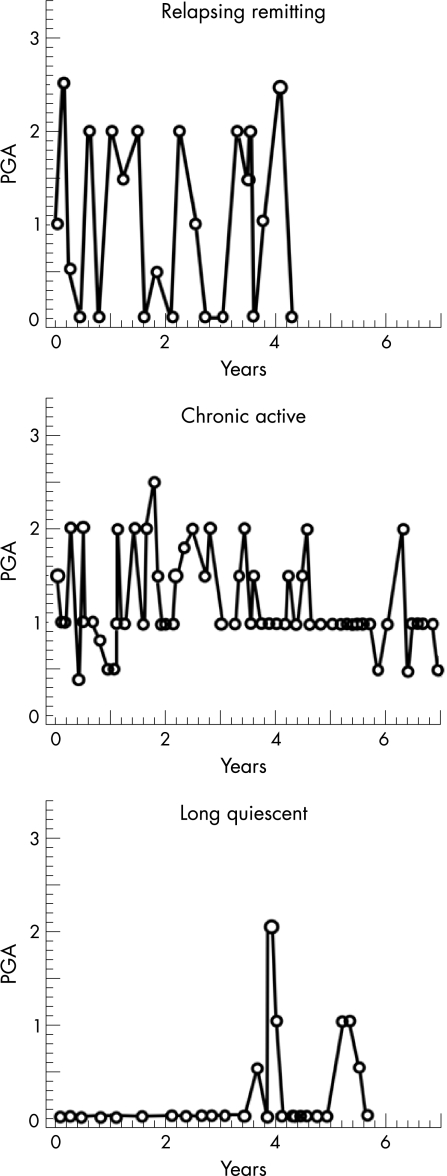
SLE is a complex disease, not just in the variety of organ system involvement, but also in the patterns of disease activity. We have identified three patterns of activity: flare, chronic activity or remission (fig 2).14 The two types of activity patterns, flare and chronic activity, should define what type of patient enters a clinical trial. For example, the “flare” patient is ideal when the outcome is reduction in flares. The “chronically active” patient is ideal when the outcome is reduction in disease activity. But not vice versa.
Figure 2 Different patterns of disease activity in SLE. Adapted from Barr et al.14 PGA, Physician Global Assessment.
Flare designs require multiple variable analyses
Not all patients with SLE have the same risk of flare. For example, patients with SLE who are female, African‐American, or who have low C3 or C4, or high anti‐dsDNA are more likely to have a flare over the next year. To balance all these factors at the baseline randomisation may be impossible. Thus, adjusting for them in the final analysis is desirable.
Conclusion
If a new therapy works, our existing disease activity indices can, and will, show it. Lupus activity indices, however, have had a very painful transition from clinical research to clinical trials. Certainly they can be improved upon, and should be. However, many of the issues bedevilling lupus trials involve other issues, such as choice of outcome, choice of study design and selection of patients. These issues deserve more attention. Ultimately, much can be said for simple trial designs with straightforward outcomes.
Abbreviations
GFR - glomerular filtration rate
SLE - systemic lupus erythematosus
Footnotes
Competing interests: None declared.
References
- 1.Stoll T, Stucki G, Malik J, Pyke S, Isenberg D A. Further validation of the BILAG disease activity index in patients with systemic lupus erythematosus. Ann Rheum Dis 199655756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand V, Gladman D, Isenberg D, Petri M, Smolen J, Tugwell P. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol 199926490–497. [PubMed] [Google Scholar]
- 3.Ward M M, Marx A S, Barry N N. Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus. J Rheumatol 200027664–670. [PubMed] [Google Scholar]
- 4.Chang E, Abrahamowicz M, Ferland D, Fortin P R. Comparison of the responsiveness of lupus disease activity measures to changes in systemic lupus erythematosus activity relevant to patients and physicians. J Clin Epidemiol 200255488–497. [DOI] [PubMed] [Google Scholar]
- 5.Ho A, Magder L, Barr S, Petri M. Decreases in anti‐double stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum 2001442342–2349. [DOI] [PubMed] [Google Scholar]
- 6.Gladman D D, Urowitz M B, Kagal A, Hallett D. Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J Rheumatol 200027377–379. [PubMed] [Google Scholar]
- 7.Petri M, Kim M Y, Kalunian K C, Grossman J, Hahn B H, Sammaritano L R.et al Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 20053532550–2558. [DOI] [PubMed] [Google Scholar]
- 8.Rahman P, Humphrey‐Murto S, Gladman D D, Urowitz M B. Cytotoxic therapy in systemic lupus erythematosus. Experience from a single center. Medicine (Baltimore) 199776432–437. [DOI] [PubMed] [Google Scholar]
- 9.Remer C F, Weisman M H, Wallace D J. Benefits of leflunomide in systemic lupus erythematosus: a pilot observational study. Lupus 200110480–483. [DOI] [PubMed] [Google Scholar]
- 10.Chan T M, Li F K, Tang C S, Wong R W, Fang G X, Ji Y L.et al Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong‐Guangzhou Nephrology Study Group. N Engl J Med 20003431156–1162. [DOI] [PubMed] [Google Scholar]
- 11.Ginzler E M, Dooley M A, Aranow C, Kim M Y, Buyon J, Merrill J T.et al Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 20053532219–2228. [DOI] [PubMed] [Google Scholar]
- 12.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O'Nan P.et al Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004350971–980. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Hydroxychloroquine Study Group A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991324150–154. [DOI] [PubMed] [Google Scholar]
- 14.Barr S, Zonana‐Nacach A, Magder L, Petri M. Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum 1999422682–2688. [DOI] [PubMed] [Google Scholar]