Abstract
Identification of the factors that regulate the immune tolerance and control the appearance of exacerbated inflammatory conditions is crucial for the development of new therapies of autoimmune diseases. Some neuropeptides and hormones have emerged as endogenous agents that participate in the regulation of the processes that ensure self‐tolerance. Among them, the vasoactive intestinal peptide (VIP), a well‐characterised endogenous anti‐inflammatory neuropeptide, has shown therapeutic potential for a variety of immune disorders. Here we examine the latest research findings, which indicate that VIP participates in maintaining immune tolerance in two distinct ways: by regulating the balance between pro‐inflammatory and anti‐inflammatory factors, and by inducing the emergence of regulatory T cells with suppressive activity against autoreactive T cell effectors.
The successful elimination of most pathogens requires crosstalk between the innate and adaptive arms of the immune system. The innate immune system recognises pathogen‐associated molecular patterns through pattern‐recognition receptors, such as toll‐like receptors (TLRs), which induce the release of pro‐inflammatory cytokines, chemokines and free radicals, recruitment of inflammatory cells to the site of infection, and lysis of infected host cells by natural killer cells and cytotoxic T lymphocytes. However, further damage can arise from potential autoimmune responses occurring during the inflammatory response, in which the immune cells and molecules that respond to pathogen‐derived antigens also react to self‐antigens. Therefore, in an inflammatory and autoimmune disease like rheumatoid arthritis, the initial stages involve multiple steps that can be divided into two main phases: early events associated with initiation and establishment of autoimmunity to joint components in peripheral lymphoid organs, and later events associated with the evolving immune and destructive inflammatory responses in the joint (fig 1).1 Progression of the autoimmune response involves the development of self‐reactive T helper 1 (Th1) cells, their entry into the joint, release of pro‐inflammatory cytokines and chemokines, subsequent recruitment and activation of inflammatory cells (macrophages, neutrophils and mast cells) and synovial pannus formation. Production of inflammatory mediators, such as cytokines, matrix‐degrading enzymes and free radicals by infiltrating cells and resident synovial cells (fibroblasts and osteoclasts) damages cartilage and bone. In addition, Th1‐mediated production of autoantibodies by B cells, which form immune complexes and activate complement and neutrophils, contribute to autoimmune pathology and disease propagation.
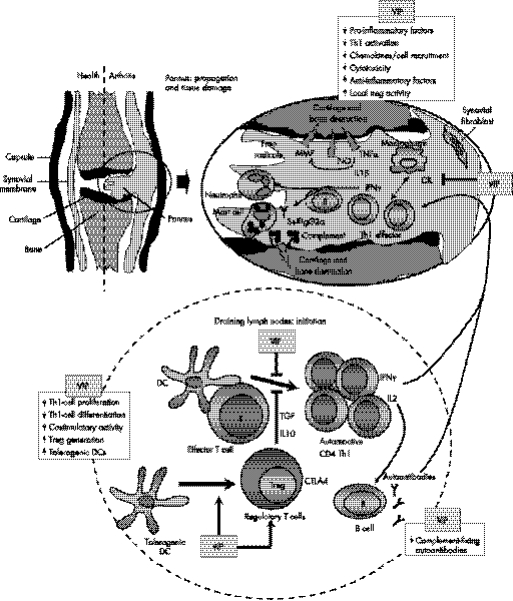
Figure 1 VIP restores tolerance in autoimmune disorders by acting at multiple levels. Loss of immune tolerance compromises immune homeostasis and results in the onset of autoimmune disorders. This figure illustrates one scenario depicting the stepwise progression of the development of rheumatoid arthritis. The initial stages of the disease take place in peripheral lymphoid organs, are associated with the initiation and establishment of autoimmunity to joint components, and involve the development of self‐reactive T helper 1 (Th1) cells by dendritic cells (DCs) presenting self‐antigens. In addition, Th1 cells induce the production of IgG2 autoantibodies by autoreactive B cells and the deposition of immune complexes in the joint. Progression of the autoimmune response involves the entry of autoreactive Th1 cells into the joint, release of pro‐inflammatory cytokines (TNFα and interferon γ) and chemokines, and subsequent recruitment and activation of inflammatory cells (macrophages and neutrophils). In addition, autoantibodies activate complement, neutrophils and mast cells. Later events are associated with the evolving immune and destructive inflammatory responses. Inflammatory mediators, such as cytokines, nitric oxide (NO), free radicals and metalloproteinases (MMP), which are produced by infiltrating cells and resident synovial cells, have a crucial role in cartilage and bone destruction. Naturally occurring CD4 CD25+ T regulatory (Treg) cells or induced regulatory T cells are key players in maintaining tolerance by their suppression of self‐reactive Th1 cells. Imbalance of regulatory T cells versus Th1 cells, or of anti‐inflammatory cytokines vs pro‐inflammatory factors, are the cause of autoimmune disorders. VIP induces immune tolerance and inhibits the autoimmune response through different non‐excluding mechanisms. (A) VIP decreases Th1 cell functions through direct actions on differentiating T cells, or indirectly by regulating DC functions. The inflammatory and autoimmune responses are impaired because the infiltration/activation of neutrophils and macrophages and deposition of immune complexes are avoided. (B) VIP inhibits the production of inflammatory cytokines, chemokines and free radicals by macrophages and synoviocytes, avoiding the inflammatory response and its cytotoxic effect against joint components. (C) VIP induces the new generation of peripheral Treg cells that suppress activation of autoreactive T cells through a mechanism that involves production of interleukin 10 (IL10) and transforming growth factor‐β (TGFβ), and/or expression of the cytotoxic T lymphocyte‐associated antigen 4 (CTLA4). In addition, VIP indirectly generates Treg cells through the differentiation of tolerogenic DCs. Arrows indicate a stimulatory effect. Back‐crossed lines indicate an inhibitory effect.
Therefore, safe induction of antigen‐specific long‐term tolerance is critical for the control of autoreactive T cells on autoimmune diseases. In addition, although critical for control of infection, the inflammatory process needs to be limited, since excessive responses result in severe inflammation and collateral tissue damage. In general, inflammatory responses are self‐controlled by anti‐inflammatory mediators secreted by host innate immune system during the ongoing process, and the ability to control an inflammatory state depends on the local balance between pro‐inflammatory and anti‐inflammatory factors. Moreover, the adaptive immune system also helps to maintain immune tolerance during infection‐induced immunopathology.2 In addition to the intrinsic control of lymphocytes, for example, clonal deletion of self‐reactive T cells in the thymus via apoptosis of immature self‐reactive lymphocytes upon exposure to self‐antigen or activation‐induced cell death of mature effector cells, the generation of antigen‐specific regulatory T cells (Treg) plays a critical role in the induction of peripheral tolerance (fig 1). For example, depletion of CD4 CD25+ Treg cells produces autoimmune disease in otherwise normal animals, and their reconstitution prevents disease.2,3,4 Importantly, Treg cells have been shown to be deficient in patients with rheumatoid arthritis, multiple sclerosis, type‐1 diabetes and other autoimmune diseases.5,6,7 Moreover, numerous studies have demonstrated the therapeutic use of antigen‐specific Treg cells in various models of autoimmune disorders.2,3,4
Although the idea of Treg cells with a suppressive activity on immune responses has been around for more than two decades, the characterisation of diverse Treg populations with different developmental, phenotypical or functional characteristics has been quite recent. From a developmental point of view, it is accepted that two populations of Treg cells exist: natural (or constitutive) and inducible (or adaptive) Treg cells (table 1). Natural Treg cells develop and migrate from the thymus and constitute 5–10% of peripheral T cells in normal mice and humans. These CD4 CD25+ Treg cells express the transcriptional repressor FoxP3 and cytotoxic T‐lymphocyte antigen 4 (CTLA4). Natural Treg cells suppress clonal expansion of self‐reactive T cells through a mechanism that is cell–cell contact mediated by CTLA4, which interacts with CD80 and/or CD86 on the surface of the antigen‐presenting cells (APCs) and delivers a negative signal for T cell activation. In vivo, but not in most studies in vitro, a role for cytokines such as IL10 and TGFβ in the function of natural Treg cells has been also defined.2,3,4 Other populations of antigen‐specific Treg cells can be induced from CD4 CD25– or CD8 CD25– T cells in the periphery under the influence of tolerogenic semi‐mature dendritic cells and/or various soluble factors such as IL10, TGFβ1 and interferon α. The inducible Treg populations consist of distinct subsets: T regulatory 1 (Tr1) cells, which secrete high levels of IL10 and probably TGFβ1; T helper 3 (Th3) cells, which secrete high levels of TGFβ1; and CD8 Treg cells, which secrete IL10. These immunosuppressive cytokines inhibit the proliferation of and cytokine production by effector T cells, as well as the cytotoxic activity of CD8 T cells, either directly or through their inhibitory action on the maturation/activation of APCs. In addition, CD8 Treg cells induce the expression of the immunoglobulin‐like transcripts ILT3 and ILT4 in APCs, which downmodulate APC function. These suppressive responses can be beneficial to restore the immune homeostasis of the host, suppressing the autoreactive Th1 responses involved in the destruction of the target tissue in autoimmune diseases, or inhibiting host CD4 and CD8 T cells that react to alloantigens that cause transplant rejection. However, these suppressive responses of Treg cells can also be detrimental because effective immune responses to infective pathogens and autologous tumour cells can be impaired.
Table 1 Types of regulatory T cells involved in immune tolerance: phenotype, function and origin.
| Treg cell type | Origin | Phenotype | Suppressive mechanism |
|---|---|---|---|
| Natural Treg cells | Thymus | CD4 CD25high FoxP3+ GITR+ CTLA4high CD45RBlow CD127low | Cell‐contact (CTLA4) dependent (most studies) and IL10/TGFβ1 (in vivo studies) |
| Expanded natural Treg cells | Expansion of natural Treg cells in periphery | CD4 CD25high FoxP3+ CD69+ | Cell‐contact (CTLA4) dependent |
| Th3 cells | Periphery | CD4 FoxP3+/‐ | TGFβ1 |
| Induced Treg cells | Generation and/or expansion of non‐regulatory CD4 T cells | CD4 CD25high FoxP3+ GITR+ CTLA4high CD45RBlow | Cell‐contact (CTLA4) dependent and in some cases TGFβ1 |
| Tr1 cells | Induced by tolerogenic DCs in the periphery | CD4 CD25+FoxP3+/‐ | IL10 and/or TGFβ1 |
| CD8 Treg cells | Induced by tolerogenic DCs in the periphery | CD8 CD28+/– | IL10, cell‐contact dependent, ILT3 and ILT4 |
For simplicity, a consensus of the most widely accepted characteristic of Treg cells is shown.
CTLA4, cytotoxic T‐lymphocyte antigen 4; DCs, dendritic cells; GITR, glucocorticoid‐induced tumour necrosis factor receptor‐related protein; ILT, immunoglobulin‐like transcript; TGFβ1, transforming growth factor β1; Tr1, T regulatory 1.
From a therapeutic point of view, the fact that the appearance of exacerbated inflammatory and autoimmune diseases is a consequence of an imbalance in pro‐inflammatory factors versus anti‐inflammatory cytokines, or in self‐reactive Th1 cells versus Treg cells, it becomes critical to identify agents that help bring these disequilibriums back to normal. One could think that endogenous factors might be produced by the immune cells during the autoimmune response in an attempt to maintain it under control. Numerous researchers have concentrated their efforts investigating traditional immunosuppressive cytokines, such as IL10, IL13 and TGFβ1.8 However, others have focused their search on neuropeptides and hormones, classically considered as neuroendocrine mediators, but which are also produced by immune cells, especially under inflammatory conditions.9,10 Among them, vasoactive intestinal peptide (VIP) has lately emerged as a potential candidate for the treatment of the unwanted immune responses that occur in inflammatory and autoimmune disorders. The therapeutic effects of VIP on immune disorders have been classically attributed to its dual capacity to downregulate the inflammatory response and to inhibit antigen‐specific Th1‐driven responses.11 Furthermore, recent data suggest that VIP might facilitate immune homeostasis through a newly discovered mechanism involving the generation of Treg cells. Here we examine the most recent developments regarding the effects of VIP on immune tolerance, and the effectiveness of using this neuropeptide in treating several autoimmune diseases.
VIP and immune tolerance
VIP is a 28‐aminoacid peptide of the secretin/glucagon family that was firstly isolated from the gastrointestinal tract by its capacity as a vasodilator.12 VIP was immediately identified in the central nervous system and peripheral nerves, and was recognised as a widely distributed neuropeptide, acting as a neurotransmitter in many tissues. The widespread distribution of VIP correlates with its involvement in a wide variety of biological activities including vasodilation, bronchodilation, hyperglycaemia and hormonal regulation. From an immunological point of view, VIP has certain characteristics that make it attractive for immune tolerance. First, VIP is produced by immune cells, mainly Th2 cells and type 2 CD8 T cells, especially under inflammatory conditions, or following antigenic stimulation.13 Second, VIP exerts its biological actions through various G‐protein‐coupled receptors (VPAC1, VPAC2 and PAC1), that are expressed on various immune cells, such as T cells, macrophages, monocytes, dendritic cells (DCs) and neutrophils.11 Third, VIP signalling involves the activation of the cAMP/protein kinase A (PKA) pathway that is considered as an immunosuppressive signal.14
Role of VIP in innate immunity: anti‐inflammatory effect
Numerous evidence demonstrate that VIP is a potent anti‐inflammatory agent in vitro and in vivo that acts at different levels. It inhibits phagocytic activity, free radical production, adherence and migration of macrophages.15 It reduces the production of inflammatory cytokines (TNFα, IL12, IL6 and IL1β) and various chemokines and downregulates the expression of inducible nitric oxide synthase and the subsequent release of nitric oxide by macrophages, DCs and microglia.11 It stimulates the production of anti‐inflammatory cytokines such as IL10 and IL1Ra.16 It decreases the co‐stimulatory activity of APCs for antigen‐specific T cells by downregulating the expression of the co‐stimulatory molecules CD80 and CD86.17 It inhibits the degranulation of mast cells.18 It also reduces the expression of TLRs and associated molecules.19,20 The definitive establishment of VIP as a natural anti‐inflammatory factor has been supported by two recent works reporting that mice that lack VIP or the PAC1 receptor show higher systemic inflammatory responses and are more susceptible to die by septic shock.21,22
Role of VIP in adaptive immunity: suppressive effect on Th1 responses
Although the differentiation into Th1 or Th2 effector cells mainly depends on the nature of the APCs and the cytokine microenvironment, the involvement of other endogenous factors, such as VIP, in the regulation of the Th1/Th2 balance has been recently proposed. Macrophages and DCs treated in vitro with VIP induce Th2‐type cytokines (IL4 and IL5) and inhibit Th1‐type cytokines (IFNγ, IL2) in antigen‐primed CD4 T cells.17,23 In addition, VIP administration to immunised mice resulted in a decreased number of IFNγ‐secreting cells and an increased number of IL4‐secreting cells.23 Correspondingly, VIP receptor‐deficient mice have increased Th1‐type responses (ie, delayed‐type hypersensitivity), whereas mice that overexpress VIP receptors show eosinophilia, high levels of IgE and IgG1, and increased cutaneous anaphylaxis (typical Th2‐type responses).24,25 In addition, it has been demonstrated that the endogenous Th2‐cell‐derived VIP maintains the Th2 bias, in a positive feedback.26
Although the precise mechanisms remain to be elucidated, VIP appears to regulate the Th1/Th2 balance in several ways. First, VIP inhibits the production of the Th1‐associated cytokine IL12.27 Second, VIP induces CD86 expression in resting murine DCs, which is important for the development of Th2 cells.17,23 Third, VIP has been shown to promote specific Th2‐cell recruitment by inhibiting CXC‐chemokine ligand 10 (CXCL10) production and inducing CC‐chemokine ligand 22 (CCL22) production, two chemokines that are involved in the homing of Th1 cells and Th2 cells, respectively.28,29 Fourth, VIP inhibits CD95 (FasL)‐mediated and granzyme B‐mediated apoptosis of mouse Th2 but not of Th1 effector cells.30,31 Finally, VIP induces the Th2 master transcription factors c‐MAF, GATA‐3 and JUNB in differentiating murine CD4 T cells, and inhibits T‐bet, which is required for Th1 cell differentiation.32,33 Thus VIP regulates the Th1/Th2 balance by acting directly on differentiating T cells and indirectly via the regulation of APC functions.
Role of VIP in Treg cells: generation of a mixture of Treg cells
Recently, various reports have shown that VIP promotes tolerance by inducing the peripheral expansion of antigen‐specific Treg cells. We found that the administration of VIP together with specific antigen to T cell receptor‐transgenic mice results in the expansion of the CD4 CD25+ FoxP3+ T cells, which inhibit responder T cell proliferation through direct cellular contact.34 In addition, to increase the number of CD4 CD25+ Treg cells, VIP induces more efficient suppressors on a per‐cell basis. Transfer of these Treg cells expanded by VIP induces antigen‐specific suppression to naive hosts, inhibiting delayed‐type hypersensibility and antibody production.34 The VIP‐induced generation of Treg cells is especially evident under autoimmune conditions. Thus, VIP treatment of experimental autoimmune encephalomyelitis (EAE) and arthritic mice results in a significant increase in CD4 CD25+ T‐cell numbers in draining lymph nodes, brain and joints.35,36 The VIP‐induced CD4 CD25+ cells exhibit an activated Treg cell phenotype, that is, CD45RBlow CD62Lhigh CD69high FoxP3high CTLA4high and produce high levels of IL10 and TGFβ1 as suppressive molecules.35,36 We now should ask about the type(s) of Treg cells induced by VIP, and the mechanism involved in such induction. According to table 1, a variety of possibilities exists. VIP could induce the de novo generation of natural CD4 CD25+ Treg cells in the thymus, or of some type(s) of inducible Treg cells (Tr1, Th3 or CD8) from naive peripheral T cells. Alternatively, VIP could promote the peripheral expansion of already existing natural and/or inducible Treg cells. Several pieces of evidence indicate that VIP induces the new generation in the periphery of a mixture of Treg cells. Interestingly, the in vivo VIP‐induced CD4 CD25+ Treg cells mediate their suppressive action on autoreactive T cells by secreting suppressive soluble factors, such as IL10 and TGFβ1, and through direct cellular contact that is mainly dependent on CTLA4.34,35,36 This distinguishes the VIP‐induced Treg cells from the classical Tr1 or Th3 cells, whose suppressive mechanism is cytokine‐dependent,2,3,4 and from the natural CD4 CD25+ Treg cells or the CD4 CD25+ Treg cells generated from the peripheral CD4 CD25– T‐cell population, which are contact‐dependent and cytokine‐independent suppressors (table 1). This suggests that the Treg population induced by VIP in vivo could be a novel population, or that VIP induces or activates different types of the previously described Treg subsets which cooperatively act in the suppressive response. The latter seems to be the most plausible possibility, and VIP could induce in vivo at least two Treg populations: a major population of FoxP3+ CTLAhigh CD4 CD25+ Treg cells that resembles the reported CD25+ T cell recruited from the peripheral CD25– T cell population by IL2 and TGFβ‐activated CD4 CD25+ T cells,37 and a minor population of IL10/TGFβ‐producing Treg cells that phenotypically resembles Tr1 cells induced by tolerogenic DCs differentiated with various immunosuppressive factors.38,39 Recent studies have shown that VIP promotes the generation of tolerogenic DCs in vitro and in vivo,40,41,42,43 which induce antigen‐specific tolerance by generating Tr1‐like cells.
Regarding the major Treg population induced by VIP, although the mechanisms involved in its generation or expansion are not fully understood, our hypothesis is that VIP induces the peripheral generation of new Treg cells from the CD4 CD25– T cell repertoire. Recent evidence supports this hypothesis. First, VIP administration prevents disease progression in CD25‐depleted arthritic and EAE mice by inducing the emergence of peripheral CD4 CD25+ Treg cells.35,36 Second, VIP generates in vitro CTLA4+ FoxP3+ CD4 CD25+ Treg cells from CD4 CD25– T cells isolated from arthritic mice.36 In any case, we cannot rule out that VIP participates in the generation of natural thymic Treg cells, without affecting their peripheral expansion. It is still unknown whether VIP induces the generation of FoxP3+ CD4 CD25+ Treg cells from all the CD4 CD25– T cell repertoire, or whether it specifically affects only a proportion of them which are already committed to generate Treg cells. If this is the case, VIP could be simply expanding this Treg‐committed CD4 CD25– T population.
Finally, whether VIP is able to induce CD8 Treg cells is still unknown. However, we have found that, similarly to activated plasmacytoid cells,44 the tolerogenic DCs generated with VIP from human monocytes induce antigen‐specific IL10‐producing CD8 CD28– CTLA4+ Tr1‐like cells in vitro,43 suggesting that VIP could induce CD8 Treg cells in vivo, at least indirectly through tolerogenic DCs.
Therapeutic effect of VIP on autoimmunity
The capacity of VIP to regulate a wide spectrum of inflammatory factors and to switch the Th1/Th2 balance in favour of Th2 immunity make it an attractive therapeutic candidate for the treatment of inflammatory disorders and/or Th1‐type autoimmune diseases. Indeed, administration of VIP delays the onset, decreases the frequency and reduces the severity of various experimental models of sepsis,18,45 rheumatoid arthritis,46,47,48,49 Crohn's disease,50 type‐1 diabetes,32,51 multiple sclerosis,52,53 Sjogren's syndrome,54 pancreatitis,55 keratitis56 and uveoretinitis.57 The therapeutic effect of VIP is associated with the reduction of the two main phases of these immune disorders. VIP treatment impairs early events that are associated with the initiation and establishment of autoimmunity to self‐tissue components, as well as later phases that are associated with the evolving immune and destructive inflammatory responses. VIP reduces the development of self‐reactive Th1 cells, their entry into the target organ, the release of pro‐inflammatory cytokines (mainly TNFα and IFNγ) and chemokines, and the subsequent recruitment and activation of macrophages and neutrophils (fig 1). This results in a decreased production of destructive inflammatory mediators (cytokines, nitric oxide, free radicals and matrix metalloproteinases) by infiltrating and resident (ie, microglia or synoviocytes) inflammatory cells. In addition, the inhibition of the self‐reactive Th1‐cell response by VIP gives a decreased titre of IgG2a autoantibodies, which activate complement and neutrophils and contribute to tissue destruction. Induction of Treg cells by VIP is the latest piece added to this puzzle. The involvement of these Treg cells in the beneficial effect of VIP on autoimmunity is supported by the fact that the in vivo blockade of the Treg cell mediators CTLA4, IL10 and TGFβ1, but not the Th2‐type cytokine IL4, significantly reversed the therapeutic action of VIP.35,36 Therefore, the generation of Treg cells by VIP could explain the selective inhibition of Th1 immune responses once T cells have completed differentiation into Th1 effector cells, as evidenced by the therapeutic effect of delayed administration of VIP in established arthritis, EAE and diabetes.46,51,52
Is VIP ready for the clinic?
The findings reviewed above indicate that VIP acts in a pleiotropic and in many cases redundant manner to regulate the balance between pro‐inflammatory and anti‐inflammatory factors, and between autoreactive Th1 cells and Treg cells (fig 1). Based on these characteristics, VIP appears to represent an exciting prospect as a therapeutic agent for the treatment of immune diseases, such as rheumatoid arthritis, type‐1 diabetes, multiple sclerosis, Crohn's disease and other diseases characterised by both inflammatory and autoimmune components. Whereas VIP strongly ameliorates all these organ‐specific autoimmune disorders, the effect of VIP on systemic autoimmune diseases has still not been investigated. However, autoantibodies against VIP have been found in animals and patients with systemic lupus erythematosus,58 suggesting that the depletion of VIP by specific antibodies in this systemic autoimmune disease may exacerbate autoreactive responses.
Induction of Treg cells by VIP has not only been crucial to a better understanding of the immunomodulatory action on VIP, but has also supported the proposal of a new cell‐based strategy for the treatment of immune disorders where tolerance restoration is needed. Considerable effort has recently been focused on the use of antigen‐specific Treg cells generated ex vivo for the treatment of autoimmune diseases, transplantation and asthmatic disorders.3 The ability to translate important biological findings about Treg cells to the clinic has been limited by several issues, including the low frequency of these cells and the potential for pan immunosuppression. A potential solution to this problem could be expanding Treg cells and making them antigen‐specific using selected antigens and peptides. However, although Treg cells replicate relatively efficiently in vivo, they are anergic and refractory to stimulation in vitro.4,59,60 Therefore, protocols that efficiently expand Treg populations in vitro while maintaining their immunoregulatory properties in vivo should be based in the conditions that allow their expansion in vivo, including T cell receptor (TCR) occupancy, crucial co‐stimulatory signals and selective growth factors. VIP could be one of the endogenous growth factors involved in the generation/expansion of Treg cells. In fact, VIP induces the generation of self‐peptide‐specific Treg cells from otherwise conventional T cells in vitro.35,61 These cells prevent very efficiently the progression of experimental autoimmune diseases by suppressing the systemic autoantigen‐specific T and B cell responses and the tissue‐localised inflammatory response.35,36 On the other hand, the capacity of certain classes of DCs to induce Treg cells makes them attractive for the expansion/generation of antigen‐specific Treg cells ex vivo, or alternatively, for their use in vivo as therapeutic cells that restore immune tolerance by inducing Treg cells in the host.38,39 In this sense, VIP‐induced tolerogenic DCs pulsed with self‐antigens have been shown to ameliorate the progression of rheumatoid arthritis, EAE and inflammatory bowel disease.40,41 This effect is mainly mediated through the generation of antigen‐specific Tr1‐like cells in the treated animal.
VIP shows therapeutic advantages versus agents directed against only one component of these diseases, for which combinatory therapies have been proposed by other researchers. However, most studies describing the therapeutic potential of VIP that have been carried out so far have been performed using animal models, and although valuable, these findings should be extended to human diseases with caution. Differences may be expected in terms of peptide dosage and in the expression of specific receptors by different immunocompetent cells. However, it is important to note that VIP has previously been tested in humans for the treatment of sepsis and other disorders,62 (see NCT00004494 clinical trial: http://www.ClinicalTrials.gov], suggesting that it should be well tolerated in humans in doses similar to those that are able to prevent immunological diseases in animals, with no side effects such as excessive vasodilation, effects on neural function or hormone imbalance.
Despite these advantages, several obstacles stand between translating VIP‐based treatment into viable clinic therapies. Due to its natural structural conformation, VIP is very unstable and extremely sensitive to the peptidases present in most tissues. Several strategies have been developed to increase VIP half‐life such as modification and/or substitution of certain amino acids in the sequence or cycling the structure, which increases the stability of the peptide.63,64 More important is work towards improving neuropeptide delivery to target tissues and cells while protecting it against degradation, including neuropeptide gene delivery or the insertion of VIP into micelles or nanoparticles.51,54,62,65,66 Other strategies combine VIP treatment with inhibitors of neutral endopeptidases to reduce the degradation of the peptide in the circulation.67 Other combinatory treatments aim to take advantage of the fact that activation of the cAMP/PKA pathway appears to be the major signal involved in the VIP immunomodulatory effect, thus combining VIP with inhibitors of phosphodiesterases (enzymes involved in the degradation of cAMP) has been found to be therapeutically attractive in the treatment of some inflammatory diseases.68
Regarding the cell‐based therapy proposed with VIP‐induced Treg cells or tolerogenic DCs, probably the most important issue that needs to be resolved is to determine the necessity of antigen specificity. Whereas polyclonal Treg cells might function in allograft transplantation and autoimmunity in lymphopaenic (ie, systemic lupus erythematosus) or inflammatory bowel disease settings, in other autoimmune disorders, antigen‐specific Treg cells are most effective.3 In this sense, aetiology and self‐antigens in most human autoimmune disorders are mostly unknown, reducing the therapeutic efficiency and applicability of Treg cells. In addition, the proposed cell‐based therapy will require an ex vivo manipulation of the blood cells of patients. Therefore, it is necessary to determine whether VIP in vitro is able to generate Treg cells or tolerogenic DCs with the same efficiency, reliability, homing capacity and survival in vivo as those obtained from animals or healthy subjects, since in contrast to mouse models, in patients there exists considerable variability. In any case, what we are proposing is an individualised therapy, which will involve procedures that are likely to be expensive, but which will be indicated to patients that are non‐responsive to established treatments.
However, the principal approach of the pharmaceutical companies, as a prerequisite for successful clinical applications, is the development of metabolically stable analogues. Understanding of the structure/function relationship of VIP and its specific receptors, including receptor signalling, internalisation and homo/heterodimerisation, will be essential for the development of novel pharmacological agents for the treatment of inflammatory/autoimmune disorders and opening up new applications for VIP‐derived treatments. However, in the case of the type 2 G protein‐coupled receptors (ie, receptors for VIP, urocortin, melanocyte‐stimulating hormone and adrenomedullin), the pharmaceutical industry has so far failed to generate effective non‐peptide‐specific agonists. Even where synthetic agonists were designed specifically for VIP receptors, they were less effective than the natural peptide as anti‐inflammatory agents.45,46,50,52 In any case, the focus on the use of natural peptides in therapy is not new and may be a case of history repeating itself, since naturally occurring human compounds have often proved to have striking therapeutic value (eg, insulin and cortisone). It is significant that the organism responds to an exacerbated inflammatory response by increasing the peripheral production of endogenous anti‐inflammatory neuropeptides,9,10 in an attempt to restore the immune homeostasis.
Abbreviations
APCs - antigen‐presenting cells
DCs - dendritic cells
PKA - protein kinase A
TLRs - toll‐like receptors
Treg - T regulatory
Tr1 - T regulatory 1
VIP - vasoactive intestinal peptide
Footnotes
Funding: This work was supported by grants from the Spanish Ministry of Health, the NIH and Junta de Andalucia.
Competing interests: None declared.
References
- 1.Firestein G S. Evolving concepts of rheumatoid arthritis. Nature 2003423356–361. [DOI] [PubMed] [Google Scholar]
- 2.Mills K H G. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol 20044841–855. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone J A. Regulatory T‐cell therapy: is it ready for the clinic? Nat Rev Immunol 20055343–349. [DOI] [PubMed] [Google Scholar]
- 4.Shevach E M. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 200625195–201. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenstein M R, Evans J G, Singh A, Moore S, Warnes G, Isenberg D A.et al Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti‐TNFα therapy. J Exp Med 2004200277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindley S, Dayan C M, Bishop A, Roep B O, Peakman M, Tree T I. Defective suppressor function in CD4+CD25+ T‐cells from patients with type 1 diabetes. Diabetes 20055492–99. [DOI] [PubMed] [Google Scholar]
- 7.Viglietta V, Baecher‐Allan C, Weiner H L, Hafler D A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004199971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Y Y, Favell R A. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev 2006212114–130. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez‐Rey Ë, Chorny A, Delgado M. Regulation of immune tolerance by anti‐inflammatory neuropeptides. Nat Rev Immunol 2007752–63. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez‐Rey E, Delgado M. Anti‐inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007. doi: 10.1016/j.tips.2007.07.001/ [DOI] [PubMed]
- 11.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 200456249–290. [DOI] [PubMed] [Google Scholar]
- 12.Said S I, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science 19701691217–1218. [DOI] [PubMed] [Google Scholar]
- 13.Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol 20011662907–2912. [DOI] [PubMed] [Google Scholar]
- 14.Banner K H, Trevethick M A. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci 200425430–436. [DOI] [PubMed] [Google Scholar]
- 15.De la Fuente M, Delgado M, Gomariz R P. VIP modulation of immune cell functions. Adv Neuroimmunol 1996675–91. [DOI] [PubMed] [Google Scholar]
- 16.Delgado M, Muñoz‐Elías E J, Gomariz R P, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide enhance IL‐10 production by murine macrophages: in vitro and in vivo studies. J Immunol 19991621707–1716. [PubMed] [Google Scholar]
- 17.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affect immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity of CD4+ T cells. J Leukoc Biol 2004751122–1130. [DOI] [PubMed] [Google Scholar]
- 18.Tuncel N, Tore F, Sahinturk V, Ak D, Tuncel M. Vasoactive intestinal peptide inhibits degranulation and changes granular content of mast cells: a potential therapeutic strategy in controlling septic shock. Peptides 20002181–89. [DOI] [PubMed] [Google Scholar]
- 19.Delgado M, Leceta J, Abad C, Martinez C, Ganea D, Gomariz R P. Shedding of membrane‐bound CD14 from lipopolysaccharide‐stimulated macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide. J Neuroimmunol 19999961–71. [DOI] [PubMed] [Google Scholar]
- 20.Gomariz R P, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F.et al Time‐course expression of Toll‐like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol 200578491–502. [DOI] [PubMed] [Google Scholar]
- 21.Martinez C, Abad C, Delgado M, Arranz A, Juarranz M G, Rodrigurez‐Henche N.et al Anti‐inflammatory role in septic shock of pituitary adenylate cyclase‐activating polypeptide receptor. Proc Natl Acad Sci USA 2002991053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szema A M, Hamidi S A, Lyubsky S, Dickman K G, Mathew S, Abdel‐Razek T.et al Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol 2006291880–886. [DOI] [PubMed] [Google Scholar]
- 23.Delgado M, Leceta J, Gomariz R P, Ganea D. VIP and PACAP stimulate the induction of Th2 responses by upregulating B7.2 expression. J Immunol 19991633629–3635. [PubMed] [Google Scholar]
- 24.Voice J K, Dorsam G, Lee H, Kong Y, Goetzl E J. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible VIP receptor. FASEB J 2001152489–2496. [DOI] [PubMed] [Google Scholar]
- 25.Goetzl E J, Voice J K, Shen S, Dorsam G, Kong Y, West K M.et al Enhanced delayed‐type hypersensitivity and diminished immediate type hypersensitivity in mice lacking the inducible VPAC(2) receptor for VIP. Proc Natl Acad Sci USA 20019813854–13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voice J, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl E J. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild‐type mice and T cell‐targeted type II VIP receptor transgenic mice. J Immunol 2003170308–314. [DOI] [PubMed] [Google Scholar]
- 27.Delgado M, Munoz‐Elias E J, Gomariz R P, Ganea D. VIP and PACAP inhibit IL‐12 production in LPS‐stimulated macrophages. Subsequent effect on IFNγ synthesis by T cells. J Neuroimmunol 199996167–181. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Jing H, Ganea D. VIP and PACAP down‐regulate CXCL10 (IP‐10) and up‐regulate CCL22 (MDC) in spleen cells. J Neuroimmunol 200213381–94. [DOI] [PubMed] [Google Scholar]
- 29.Delgado M, Gonzalez‐Rey E, Ganea D. VIP/PACAP preferentially attract Th2 versus Th1 cells by differentially regulating the production of chemokines by dendritic cells. FASEB J 2004181453–1455. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation‐induced cell death, is down‐regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol 200617697–110. [DOI] [PubMed] [Google Scholar]
- 31.Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J 2002161844–1846. [DOI] [PubMed] [Google Scholar]
- 32.Rosignoli F, Torroba M, Juarranz Y, Garcia‐Gomez M, Martinez C, Gomariz R P.et al VIP and tolerance induction in autoimmunity. Ann NY Acad Sci 20061070525–530. [DOI] [PubMed] [Google Scholar]
- 33.Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl E J. c‐Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein‐coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol 20041727289–7296. [DOI] [PubMed] [Google Scholar]
- 34.Delgado M, Chorny A, Gonzalez‐Rey E, Ganea D. Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo. J Leukoc Biol 2005781327–1338. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez‐Rey E, Fernandez‐Martin A, Chorny A, Delgado M. Vasoactive intestinal peptide induces CD4+CD25+ regulatory T cells with therapeutic effect on collagen‐induced arthritis. Arthritis Rheum 200654864–876. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez‐Martin A, Gonzalez‐Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol 200636318–326. [DOI] [PubMed] [Google Scholar]
- 37.Zheng S G, Wang J H, Gray J D, Soucier H, Horwitz D A. Natural and induced CD4+CD25+ cells educate CD4+CD25‐ cells to develop suppressive activity: the role of IL‐2, TGF‐β, and IL‐10. J Immunol 20041725213–5221. [DOI] [PubMed] [Google Scholar]
- 38.Hackstein H, Thomson A W. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004424–34. [DOI] [PubMed] [Google Scholar]
- 39.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 20061081435–1440. [DOI] [PubMed] [Google Scholar]
- 40.Chorny A, Gonzalez‐Rey E, Fernandez‐Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA 200510213562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez‐Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology 20061311799–1811. [DOI] [PubMed] [Google Scholar]
- 42.Delgado M, Gonzalez‐Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol 20051757311–7324. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez‐Rey E, Chorny A, Fernandez‐Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 20061073632–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilliet M, Liu Y J. Generation of CD8 T regulatory cells by CD40 ligand activated plasmacytoid dendritic cells. J Exp Med 2002195695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado M, Martinez C, Pozo D, Calvo J R, Leceta J, Ganea D.et al Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase‐activating polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF‐α and IL‐6. J Immunol 19991621200–1205. [PubMed] [Google Scholar]
- 46.Delgado M, Abad C, Martinez C, Leceta J, Gomariz R P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 20017563–568. [DOI] [PubMed] [Google Scholar]
- 47.Yin H, Cheng H, Yu M, Zhang F, Gao Y, Lin J.et al Vasoactive intestinal peptide ameliorates synovial cell functions of collagen‐induced arthritis rats by down‐regulating NF‐kappaB activity. Immunol Invest 200534153–169. [PubMed] [Google Scholar]
- 48.Zafirova Y, Yordanov M, Kalfin R. Antiarthritic effect of VIP in relation to the host resistance against Candida albicans infection. Int Immunol 2004161125–1131. [DOI] [PubMed] [Google Scholar]
- 49.Williams R O. Therapeutic effect of vasoactive intestinal peptide in collagen‐induced arthritis. Arthritis Rheum 200246271–273. [DOI] [PubMed] [Google Scholar]
- 50.Abad C, Martinez C, Juarranz M G, Arranz A, Leceta J, Delgado M.et al Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 2003124961–971. [DOI] [PubMed] [Google Scholar]
- 51.Herrera J L, Fernandez‐Montesinos R, Gonzalez‐Rey E, Delgado M, Pozo D. Protective role for plasmid DNA‐mediated VIP gene transfer in non‐obese diabetic mice. Ann NY Acad Sci 20061070337–341. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez‐Rey E, Fernandez‐Martin A, Chorny A, Martin J, Pozo D, Ganea D.et al Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: downregulation of inflammatory and autoimmune responses. Am J Pathol 20061681179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, Mei Y, Wang Y, Xu L. Vasoactive intestinal polypeptide suppressed experimental autoimmune encephalomyelitis by inhibiting T helper 1 responses. J Clin Immunol 200626430–437. [DOI] [PubMed] [Google Scholar]
- 54.Lodde B M, Baum B J, Tak P P, Illei G. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjorgre's disease. Ann Rheum Dis 200665195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kojima M, Ito T, Oono T, Hisano T, Igarashi H, Arita Y.et al VIP attenuation of the severity of experimental pancreatitis is due to VPAC1 receptor‐mediated inhibition of cytokine production. Pancreas 20053062–70. [PubMed] [Google Scholar]
- 56.Szliter E A, Lighvani S, Barret R P, Hazlett L D. Vasoactive intestinal peptide balances pro‐ and anti‐inflammatory cytokines in the Pseudomonas aeruginosa‐infected cornea and protects against corneal protection. J Immunol 20071781105–1114. [DOI] [PubMed] [Google Scholar]
- 57.Keino H, Kezuka T, Takeuchi M, Yamakawa N, Hattori T, Isui M. Prevention of experimental autoimmune uveoretinitis by vasoactive intestinal peptide. Arch Ophthalmol 20041221179–1184. [DOI] [PubMed] [Google Scholar]
- 58.Bangale Y, Karle S, Planque S, Zhou Y X, Taguchi H, Nishiyama Y.et al VIPase autoantibodies in Fas‐defective mice and patients with autoimmune disease. FASEB J 200317628–635. [DOI] [PubMed] [Google Scholar]
- 59.Walker L S, Chodos A, Aggena M, Dooms H, Abbas A K. Antigen‐dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J Exp Med 2003198249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fisson S, Darrasse‐Jeze G, Litvinova E, Septier F, Klatzmann D, Liblam R.et al Continuous activation of autoreactive CD4+CD25+ regulatory T cells in the steady state. J Exp Med 2003198737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez‐Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T‐cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med 200713241–251. [DOI] [PubMed] [Google Scholar]
- 62.Petkov V, Mosgoeller W, Ziesche R, Raderer M, Stiebellehner L, Vonbank K.et al Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest 20031111339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotthardt M, Boermann O C, Behr T M, Behe M P, Oyen W J. Development and clinical application of peptide‐based radiopharmaceuticals. Curr Pharm Des 2004102951–2963. [DOI] [PubMed] [Google Scholar]
- 64.Bolin D R, Michalewsky J, Wasserman M A, O'Donnell M. Design and development of a vasoactive intestinal peptide analog as a novel therapeutic for bronchial asthma. Biopolymers 19953757–66. [DOI] [PubMed] [Google Scholar]
- 65.Onyuksel H, Ikezaki H, Patel M, Gao X P, Rubinstein I. A novel formulation of VIP in sterically stabilized micelles amplifies vasodilation in vivo. Pharm Res 199916155–160. [DOI] [PubMed] [Google Scholar]
- 66.Sethi V, Onyuksel H, Rubinstein I. Liposomal vasoactive intestinal peptide. Methods Enzymol 2005391377–395. [DOI] [PubMed] [Google Scholar]
- 67.Sedo A, Duke‐Cohan J S, Balaziova E, Sedova L R. Dipeptidyl peptidase IV activity and/or structure homologs: contributing factors in the pathogenesis of rheumatoid arthritis? Arthritis Res Ther 20057253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foey A D, Field S, Ahmed S, Jain A, Feldmann M, Brennan F M.et al Impact of VIP and cAMP on the regulation of TNF‐alpha and IL‐10 production: implications for rheumatoid arthritis. Arthritis Res Ther 20035317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]