Abstract
Objective
To evaluate the available evidence on the efficacy and feasibility of the new concept of tight control in randomised trials in patients with rheumatoid arthritis (RA). Tight control is a treatment strategy tailored to the individual patient with RA, which aims to achieve a predefined level of low disease activity or remission within a certain period of time.
Methods
The literature database PubMed was searched and yielded four trials: the FIN‐RACo trial, the TICORA study, the BeSt study and the CAMERA study.
Results
Tight control resulted in greater improvement and a higher percentage of patients meeting the preset aim of low disease activity or remission when compared to the control intervention. In the FIN‐RACo trial, aimed at DAS28<2.6, 51% of patients in the tight control group achieved remission versus 16% in the contrast group (p<0.001). In the TICORA study, 65% of patients in the tight control group versus 16% of the contrast group achieved remission, based on DAS<1.6 (p<0.0001). In the CAMERA study, 50% of patients in the tight control group using a computer decision model achieved remission, versus 37% in the contrast group (p = 0.029). The BeSt study consisted of only tight control groups aimed at a DAS<1.6; remission was achieved in 38–46% of patients. This is higher than the range of remission in earlier trials of 13–36%.
Conclusion
Tight control aiming for low disease activity or even better still, remission, seems a promising option in treating patients with RA in clinical trials and probably also in daily practice.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes joint pain, progressive joint destruction and functional disability,1 due to the combined effect of chronic synovitis and progressive joint damage.2 Treatment of disease in the first months of synovitis is important to retard radiographic progression.3 This window of opportunity suggests that disease activity in patients with early RA is less severe, is characterised by a smaller load of inflammatory cells, and is more responsive to treatment. So aggressive treatment during this phase is more likely to succeed than is the same treatment applied later in the course of disease,4 when autoantigens from damaged joints possibly fuel the disease. Therefore it is important that RA should be treated and controlled as soon as possible after diagnosis and that this control should be maintained for as long as possible, consistent with patient safety.5,6
In the past, the traditional (pyramid model) treatment therapy started with non‐steroidal anti‐inflammatory drugs (NSAIDs). If this treatment was insufficiently effective, second‐line antirheumatic drugs or disease‐modifying antirheumatic drugs (DMARDs) were added. However, an immediate start of DMARDs proved to be more efficacious than a delayed introduction of DMARDs in the disease progress of RA.7,8 More recent therapeutic strategies are based on combinations of DMARDs to control inflammation in the critical early stages of RA.9,10,11 Glucocorticoids, which also can be considered as DMARDs because they are able to reduce the progression of joint damage, have been included in DMARD combination treatments of RA.12,13
The most current treatment strategies are combination therapies with conventional DMARDs and biologicals.14 With these therapies, improvement in signs and symptoms and also low disease activity and even remission come within reach. Overall, a paradigm shift is observed in the trials of RA: treatment is more frequently aimed at low disease activity or remission in each individual patient,15,16 instead of randomising groups into standardised therapies. This new way of treatment is called “tight control”. Tight control may be defined as a treatment strategy tailored to the disease activity of individual patients with RA with the aim of achieving a predefined level of low disease activity or preferably remission within a reasonable period of time.
The aim of this evaluation is to investigate how effective and feasible tight control is.
Methods
A PubMed search was performed to look for studies which used a predefined level of disease activity or remission as treatment aim using the following terms: rheumatoid arthritis, randomised trial, treatment strategy, low disease activity, remission and tight control. Abstracts were screened for the tight control principle. The search yielded four studies, in chronological order, the FIN‐RACo trial,17,18 the TICORA study,19 the BeSt study,20,21,22 and the CAMERA study.23 Different outcome parameters and study designs prohibited pooling of the effects of these studies. Therefore, study characteristics including the aim of the study (low disease activity or remission), time schedule, the method of evaluation of the individual patient, the adaptation of medication, and the results of the studies will be described.
Results
Table 1 gives an overview of the inclusion criteria and the different treatments used in the four studies. Except for the TICORA study, disease duration was less than 2 years, and the patients had to fulfil the American College of Rheumatology (ACR) classification criteria for RA for inclusion. Except for the CAMERA study, the inclusion criteria contained active disease and an age over 18 years.
Table 1 Characteristics of the tight control studies.
| Study | Interventions/groups | n | Medication at the start | Frequency of assessment | Inclusion criteria | Disease duration |
|---|---|---|---|---|---|---|
| FIN‐RACo | Combination therapy*Mono therapy | 9798 | SSZ, MTX, HCQ, prednSSZ ± predn | 3 months (variable)3/6 months (clinical decision/variable) | ARA criteria RA, 18–65 yr,symptoms <2 yr, active disease ⩾3 SJ and 3 of: ⩾28 mm/h ESR or ⩾19 mg/l CRP, ⩾29 min/ms, >5 SJ or >10 TJ | <2 yr |
| TICORA | Intensive management* | 55 | DMARD, i.a. steroid | 1 month (DAS) | 18–75 yr, disease duration | <5 yr |
| Routine management | 55 | DMARD mono | 3 months (clinical decision) | <5 yr, active disease (DAS>2.4) | ||
| BeSt | Sequential mono therapy* | 126 | MTX | 3 months (DAS44) | ACR criteria RA, ⩾18 yr, | ⩽2 yr |
| Step‐up combination therapy* | 121 | MTX | 3 months (DAS44) | disease duration ⩽2 yr, active | ||
| Initial combination therapy + h.d. predn*Initial combination therapy + infliximab* | 133128 | MTX, SSZ, prednMTX, infliximab | 3 months (DAS44)3 months (DAS44) | disease: ⩾6 of 66 SJ, ⩾6 of 68TJ, ⩾28 mm/h ESR, ⩾20 mmVAS global health | ||
| CAMERA | Intensive strategy group* | 151 | MTX | 1 month (computer decision program) | ACR criteria RA, >16 yr, earlyRA (<1 yr) | <1 yr |
| Conventional strategy group | 148 | MTX | 3 months (clinical decision) |
*Based on tight control treatment.
ACR criteria RA, American College of Rheumatism criteria for rheumatoid arthritis; ARA criteria RA, American Rheumatism Association criteria for rheumatoid arthritis; BeSt, Behandel Strategieën; CAMERA, Computer Assisted Management of Early Rheumatoid Arthritis; CRP, C‐reactive protein; DAS, disease activity score; DMARD, disease‐modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; FIN‐RACo, Finnish Rheumatoid Arthritis Combination Therapy; HCQ, hydroxychloroquine; h.d., high‐dose; i.a., intra‐articular; ms, morning stiffness; MTX, methotrexate; predn, prednisone; SJ, swollen joints; SSZ, sulfasalazine; TICORA, Tight Control of Rheumatoid Arthritis; TJ, tender joints; VAS, visual analogue scale; yr, year(s).
FIN‐RACo
The Finnish Rheumatoid Arthritis Combination Therapy (FIN‐RACo) study18 is a multicentre, randomised open‐parallel group treatment trial in which 195 patients were included in the period between 1993 and 1995. The goal of the study was to compare the effects of the combination therapy with DMARDs with those of mono DMARD therapy in patients with early RA. Based on the evaluation of the individual patient, medication was intensified after 3 months in the combination group if there was less than 50% improvement on two out of three variables: swollen joint score (SJC), tender joint score (TJC), and erythrocyte sedimentation rate (ESR) or C‐reactive protein (CRP). In the mono DMARD group medication was intensified if there was less than 25% improvement after 6 months. The aim of the FIN‐RACo study was remission, defined both with the ACR remission criteria,24 and as a Disease Activity Score including the 28‐joint count (DAS28)25 <2.6, and good treatment response according to criteria of the European League Against Rheumatism (EULAR).26 After 2 years, more patients in the combination group had met the ACR remission criteria (14% vs 3%, p = 0.013), the DAS28 remission criterion (51% vs 16%, p<0.001), and the criteria of a good treatment response (67% vs 27%, p<0.001) compared to the mono DMARD group. Combination therapy thus was better and not more hazardous than single treatment in induction of remission in early RA. Combination therapy as a tight control strategy in patients with early RA aiming for remission seems to be more efficacious than monotherapy.17
TICORA
The Tight Control of Rheumatoid Arthritis (TICORA) study19 is a single‐blind randomised controlled trial in which 111 patients, some with disease duration of up to 5 years, were included between 1999 and 2001. The goal of the study was to compare tight controlled treatment and routine treatment. The evaluation and strategy of medication adaptations and escalations was based on an objective disease activity score in the individual treated, tight control group and evaluated in the routine group by the subjective opinion of the clinician. The outcome of the TICORA study was the mean fall in disease activity score, the number of patients with good response (defined as a DAS28<2.4 after 2 years and a fall in this score from baseline of more than 1.2), and the percentage of patients in remission. The results of TICORA showed that the mean fall in the disease activity score was higher in the tight control group than in the routine group (−3.5 vs −1.9, p<0.0001). Compared with routine care, patients in the tight control group had a good response (82% vs 44%, p<0.0001) or remission (DAS28<1.6) (65% vs 16%, p<0.0001). As well as disease activity, radiographic disease progression, physical function and quality of life in the tight control group were also more favourable than in the routine care group, at no additional financial costs.
BeSt
The Behandel Strategieën (BeSt) study21 is a multicentre randomised open clinical trial in which 508 patients with early active RA were included between 2000 and 2002. The goal of the study was to compare four treatment strategies: sequential mono therapy, step‐up combination therapy, and initial combination therapy with either high‐dose prednisone or infliximab. Evaluation of individual patients was based on Disease Activity Score 44‐joint counts (DAS44) in all four groups.27 Medication was intensified if DAS44 exceeded 2.4 and was decreased if DAS⩽2.4 for the period of 6 months. The outcomes of the BeSt study were functional ability, radiographic joint damage, and the percentages of patients meeting the criterion of low disease activity (DAS44⩽2.4) or remission (DAS44<1.6). After 1 year patients of the initial combination therapy including either prednisone or infliximab had earlier functional improvement and less radiographic damage than those on sequential monotherapy or step‐up therapy. After 2 years of treatment, these differences seem to disappear and no statistically significant differences were seen between the groups in the percentage of patients in remission (46%, 38%, 41%, 42%, respectively; p = 0.7). It seems that in patients with early active RA, remarkable clinical improvement and suppression of joint damage progression can be achieved with frequent, objective treatment adjustments.20,22
CAMERA
The Computer Assisted Management for Early Rheumatoid Arthritis (CAMERA) study23 is a randomised prospective multicentre trial in which 299 patients with early RA were allocated between 1999 and 2003. The goal of the study was to compare intensive with conventional treatment, both strategies aiming for remission. This is the only tight control study comparing the same treatment in a tight control scheme and a conventional scheme. In the intensive (tight control) group, decisions on therapy were made more frequently and with the use of an objective computer decision program evaluation in the individual patient. The computer decision model was based on the SJC, TJC, ESR, and the Visual Analogue Scale (VAS) general well‐being. Medication was intensified if less than 20% improvement had occurred after each month of assessment, unless remission was reached. Every 3 months in the conventional treatment group a decision on medication was made by the clinician, mirroring common practice, and the medication was also intensified when clinically indicated, as judged by the attending physician. Remission was defined as no swollen joints and two out of three of the following variables: number of tender joints ⩽3, ESR ⩽20 mm/hr1st, and VAS general well‐being ⩽20 mm. After 2 years, 50% of the patients in the intensive treatment group versus 37% in the conventional group (p = 0.029) had been in remission for at least 6 months during the study. These results show that tailoring of treatment to the individual patient by a computerised decision program, aiming for remission, is more beneficial than the strategy mirroring daily practice also aiming for remission. Therefore, this strategy could be a helpful tool in daily clinical practice.
Discussion
In the present report efficacy and feasibility of tight control in randomised trials in patients with early RA is evaluated. It seems that tight control and the aims of low disease activity and remission are feasible and favourable.
Of the four studies, TICORA and CAMERA give the best insight into tight control, since they compared a tight control group with a routine or conventional group. Of these two studies, the CAMERA might offer the best comparison, because in this study the same medication in the same dosage has been used in the tight control group and the conventional groups. In contrast, all intervention groups in the BeSt study make use of the concept of tight control so there is no comparison group. In the FIN‐RACo study, the efficacy of a less intensive level of tight control was compared with that of a more intensive level; the latter yielded the best results.
In the evaluated studies different protocols in the tight control groups were used to make therapy choices and changes. In both the TICORA study and the BeSt study therapy changes were based on the DAS score. In the FIN‐RACo study as well as the CAMERA study therapy changes were based on the percentage improvement in disease activity of the individual patient. In contrast to FIN‐RACo, dose adjustments in the CAMERA study were based on a predefined scheme and were made using a computer decision program which increases objectivity. In the future, the protocol for therapy changes in studies used for tight control should be standardised, enabling comparison and pooling different study outcomes.
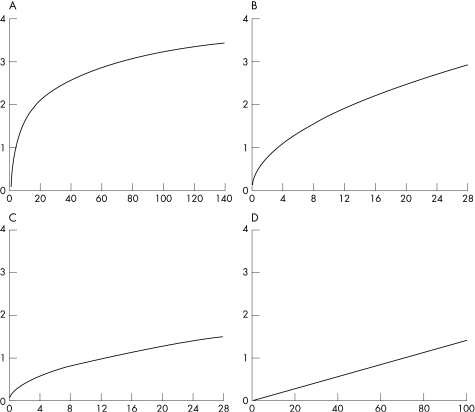
The aim in all four studies was the number of patients in remission. The range of remission rates in the tight control groups was significantly better than in the comparison groups (51–65% remission vs 16–39%) or, to make a comparison with historical controls for the groups in the BeSt study (38–46% remission), the percentages of remission in earlier trials were within a range of 13–36%.16,28,29 However, different definitions were used to define remission and tight control as a new paradigm needs the use of uniform criteria of low disease activity or remission. Sustained remission is currently, with the arrival of the combination DMARD therapy and the biologicals, an achievable goal in clinical practice.30,31 Before the main goal of treatment was to achieve remission, the ACR20/50/70 improvement criteria were standard for controlled clinical trials. But they do not give any information about the current status of disease activity. A patient could have large improvement and still have active disease.32 Criteria of low disease activity have the drawback that patients may still have progression of radiographic joint damage.33 Therefore remission rather than low disease activity should be our treatment goal today.34 On the other hand, even when remission should be the best feasible outcome measure, this term is still poorly defined and various remission criteria mirror different degrees of disease activity.31,35 In three out of four evaluated studies, the DAS27 remission criteria were used to define remission. However, the DAS has not been validated for use in individual patients. It is rather sensitive for changes in ESR in the (nearly) normal range (see fig 1).36 Figure 1 shows that the relative contribution of tender joints to the DAS28 score is much higher than that of swollen joints, which is a more specific feature of RA. The DAS could lead to overestimation of disease activity in individual patients due to the DAS components TJS and VAS general well‐being. Another problem for assessment of individual patients with RA is that the DAS28 does not include joints of the feet, which are frequently inflamed in RA. This could lead to underestimation of disease activity. The DAS28 remission criterion at a cut‐off level of 2.6 has been found to lack construct validity for use in clinical practice for this reason.37
Figure 1 Plots of contribution of individual components of the DAS28 (x‐axis) to the total DAS28 score (y‐axis). (A) ESR (0.7*LN(x)). (B) Tender joint count (0.555*SQR(x)). (C) Swollen joint count (0.284*SQR(x)). (D) Visual analogue scale patient's global assessment (0.0142*(x)). ESR, erythrocyte sedimentation rate; VAS, visual analogue scale.
In our opinion, for the practical use of tight control, a new score should be developed and validated for the assessment in individuals, enabling comparison and pooling of different study strategies. Probably the key criterion should be absence of swollen joints, because this is the key feature for RA. Further points of discussion are the duration of remission and whether or not radiographic progression should be included in remission criteria.
Overall, frequent monitoring on the basis of objective evidence of continued disease activity using a validated outcome measure and a quick, aggressive escalation protocol can improve the effects of RA considerably compared with routine care.7,38,39 Other short‐term and long‐term advantages of tight control might be the greater improvement in physical function and substantially enhanced quality of life,19 improved functional capacity,31 decline of radiographic progression,20,22 and possible cost reductions for the future.19 The disadvantages of tight control might be the frequency and intensity of the assessments, although the frequency might be high in patients with early RA but lower in established disease, and the fear of patients of overtreatment due to the aggressive escalation protocol. The solution for overtreatment might be reduction of medication in case of sustained response as applied in the BeSt and CAMERA study.
Conclusion
Tight control seems to be a promising new paradigm for reaching the aim of low disease activity, or even better remission, in clinical trials and possibly daily practice, due to a predefined decision and measurement system. For further implementation of this concept, development of a valid and easy‐to‐use criterion for remission is recommended.
Abbreviations
ACR - American College of Rheumatology
CRP - C‐reactive protein
DMARDs - disease‐modifying antirheumatic drugs
ESR - erythrocyte sedimentation rate
NSAIDs - non‐steroidal anti‐inflammatory drugs
RA - rheumatoid arthritis
SJC - swollen joint score
TJC - tender joint score
VAS - Visual Analogue Scale
Footnotes
Competing interests: None declared.
References
- 1.Scott D L, Symmons D P, Coulton B L, Popert A J. Long‐term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 198711108–1111. [DOI] [PubMed] [Google Scholar]
- 2.Pollard L, Choy E H, Scott D L. The consequences of rheumatoid arthritis: quality of life measures in the individual patient. Clin Exp Rheumatol 200523S43–S52. [PubMed] [Google Scholar]
- 3.Raza K, Buckley C E, Salmon M, Buckley C D. Treating very early rheumatoid arthritis. Best Pract Res Clin Rheumatol 200620849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum 2003481771–1774. [DOI] [PubMed] [Google Scholar]
- 5.Moreland L W, Russell A S, Paulus H E. Management of rheumatoid arthritis: the historical context. J Rheumatol 2001281431–1452. [PubMed] [Google Scholar]
- 6.Wolfe F, Cush J J, O'Dell J R, Kavanaugh A, Kremer J M, Lane N E.et al Consensus recommendations for the assessment and treatment of rheumatoid arthritis. J Rheumatol 2001281423–1430. [PubMed] [Google Scholar]
- 7.Smolen J S, Aletaha D, Machold K P. Therapeutic strategies in early rheumatoid arthritis. Best Pract Res Clin Rheumatol 200519163–177. [DOI] [PubMed] [Google Scholar]
- 8.van der Heide A, Jacobs J W, Bijlsma J W, Heurkens A H, van Booma‐Frankfort C, Van der Veen M J.et al The effectiveness of early treatment with “second‐line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996124699–707. [DOI] [PubMed] [Google Scholar]
- 9.Capell H A, Madhok R, Porter D R, Munro R A, McInnes I B, Hunter J A.et al Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double‐blind placebo‐controlled MASCOT study. Ann Rheum Dis 200766235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landewe R B, Boers M, Verhoeven A C, Westhovens R, van de Laar M A, Markusse H M.et al COBRA combination therapy in patients with early rheumatoid arthritis: long‐term structural benefits of a brief intervention. Arthritis Rheum 200246347–356. [DOI] [PubMed] [Google Scholar]
- 11.Wilske K R, Healey L A. Remodeling the pyramid: a concept whose time has come. J Rheumatol 198916565–567. [PubMed] [Google Scholar]
- 12.Bijlsma J W, Hoes J N, Van Everdingen A A, Verstappen S M, Jacobs J W. Are glucocorticoids DMARDs? Ann N Y Acad Sci 20061069268–274. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan J R, Bijlsma J W, Boers M, Shea B J. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev. 2007;CD006356 [DOI] [PMC free article] [PubMed]
- 14.van der Heijde D, Klareskog L, Rodriguez‐Valverde V, Codreanu C, Bolosiu H, Melo‐Gomes J.et al Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two‐year clinical and radiographic results from the TEMPO study, a double‐blind, randomized trial. Arthritis Rheum 2006541063–1074. [DOI] [PubMed] [Google Scholar]
- 15.Fransen J, Moens H B, Speyer I, van Riel P L. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005641294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson B, Schaufelberger C, Teleman A, Theander J. Remission and response to early treatment of RA assessed by the Disease Activity Score. BARFOT study group. Better Anti‐rheumatic Farmacotherapy. Rheumatology (Oxford) 2000391031–1036. [DOI] [PubMed] [Google Scholar]
- 17.Makinen H, Kautiainen H, Hannonen P, Mottonen T, Leirisalo‐Repo M, Laasonen L.et al Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol 200734316–321. [PubMed] [Google Scholar]
- 18.Mottonen T, Hannonen P, Leirisalo‐Repo M, Nissila M, Kautiainen H, Korpela M.et al Comparison of combination therapy with single‐drug therapy in early rheumatoid arthritis: a randomised trial. FIN‐RACo trial group. Lancet 19993531568–1573. [DOI] [PubMed] [Google Scholar]
- 19.Grigor C, Capell H, Stirling A, McMahon A D, Lock P, Vallance R.et al Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single‐blind randomised controlled trial. Lancet 2004364263–269. [DOI] [PubMed] [Google Scholar]
- 20.Allaart C F, Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Breedveld F C, Dijkmans B A, Group F S. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol 200624S077–S082. [PubMed] [Google Scholar]
- 21.Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Allaart C F, van Zeben D, Kerstens P J, Hazes J M.et al Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005523381–3390. [DOI] [PubMed] [Google Scholar]
- 22.Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Allaart C F, van Zeben D, Kerstens P J, Hazes J M.et al Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 2007146406–415. [DOI] [PubMed] [Google Scholar]
- 23. Verstappen S M, Jacobs J W, Van D, V, Heurkens A H, Schenk Y, Ter Borg E J.et al Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA). Ann Rheum Dis. 2007. Accepted for publication [DOI] [PMC free article] [PubMed]
- 24.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel A M, Prevoo M L, van't Hof M A, van Rijswijk M H, van de Putte L B, van Riel P L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 19963934–40. [DOI] [PubMed] [Google Scholar]
- 27.van der Heijde D M, van't Hof M, van Riel P L, van de Putte L B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 199320579–581. [PubMed] [Google Scholar]
- 28.Mottonen T, Paimela L, Leirisalo‐Repo M, Kautiainen H, Ilonen J, Hannonen P. Only high disease activity and positive rheumatoid factor indicate poor prognosis in patients with early rheumatoid arthritis treated with “sawtooth” strategy. Ann Rheum Dis 199857533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teir J, Gray J, Pendlebury A, Grennan D M. Outcome of patients with early rheumatoid arthritis over a two year period. Ann Rheum Dis 199958323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combe B, Landewe R, Lukas C, Bolosiu H D, Breedveld F, Dougados M.et al EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 20076634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mierau M, Schoels M, Gonda G, Fuchs J, Aletaha D, Smolen J S. Assessing remission in clinical practice. Rheumatology (Oxford) 200746975–979. [DOI] [PubMed] [Google Scholar]
- 32.Ranganath V K, Khanna D, Paulus H E. ACR remission criteria and response criteria. Clin Exp Rheumatol 200624S014–S021. [PubMed] [Google Scholar]
- 33.Molenaar E T, Voskuyl A E, Dinant H J, Bezemer P D, Boers M, Dijkmans B A. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 20045036–42. [DOI] [PubMed] [Google Scholar]
- 34.Smolen J S, Aletaha D. What should be our treatment goal in rheumatoid arthritis today? Clin Exp Rheumatol 200624S007–S013. [PubMed] [Google Scholar]
- 35.Makinen H, Hannonen P, Sokka T. Definitions of remission for rheumatoid arthritis and review of selected clinical cohorts and randomised clinical trials for the rate of remission. Clin Exp Rheumatol 200624S022–S028. [PubMed] [Google Scholar]
- 36.van der Heijde D M, Jacobs J W. The original “DAS” and the “DAS28” are not interchangeable: comment on the articles by Prevoo et al. Arthritis Rheum 199841942–945. [DOI] [PubMed] [Google Scholar]
- 37.Landewe R, van der Heijde D, van der Linden S, Boers M. Twenty‐eight‐joint counts invalidate the DAS28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis 200665637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emery P. Treatment of rheumatoid arthritis. BMJ 2006332152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidler H, Hulsemann J L. How should best strategy and tight control be translated into clinical practice? Comment on the article by Goekoop‐Ruiterman et al. Arthritis Rheum 2006542338–2339.16802376 [Google Scholar]