Abstract
T cells from patients with systemic lupus erythematosus exhibit a notable array of defects that probably contribute to the origin and development of the disease. Such abnormalities include an abnormal response to stimulation, aberrant expression of molecules that play key roles in intracellular signalling pathways, altered transcription factor activation and binding, and skewed gene expression. The combination of these alterations leads the cell to the expression of a particular phenotype that intense research has gradually uncovered over the last years. The aim of this article is to review the findings that have allowed us to better understand the behaviour of the lupus T cell and highlight the molecules that represent potential therapeutic targets.
T cells play a central role in the development and regulation of immune responses. They stimulate and suppress through mechanisms that involve direct and indirect actions on several cells, including antigen‐presenting cells and B lymphocytes. Such faculty allows them to regulate the specificity, and also the magnitude and duration of most immune responses.1 T cells from patients with systemic lupus erythematosus (SLE), a generalised autoimmune disease, exhibit a notable array of defects that probably contribute to the origin and the development of the disease.2 Several abnormalities that have been consistently reported in T cells obtained from patients with SLE have allowed us to craft a conceptual model that describes the odd behaviour of the lupus T cell and partly explains its defects.3 Evidence indicates that it has several phenotypic and functional characteristics that distinguish it from normal T cells. However, the presence of different aberrations is heterogeneous and different patients are expected to have different combinations of such defects. Thus, it is probable that each patient with SLE has a distinctive arrangement of alterations that could make her prone to specific disease manifestations and simultaneously, candidate to different therapies. In this sense, the concepts that have arisen during the last decade are of paramount importance and will permit us to understand the disease pathogenesis and to be able to design rational targeted therapies adjusted to the particularities of each individual patient. The aim of this article is to review such findings and their conceptual meaning, and highlight the molecules that represent potential therapeutic targets.
Abnormal T cell activation mechanisms
T cells from patients with SLE behave in an abnormal manner when stimulated through their specific receptor (TCR). Such phenotype includes a lower excitation threshold as well as an abnormally high intracellular calcium response. The increment in the intracellular calcium levels is more rapid and reaches a higher level than in normal T cells. This was one of the first T cell abnormalities reported to be specific to lupus T lymphocytes.4 The aberrant kinetics of the calcium response parallels an increase, in magnitude and speed, in the tyrosine phosphorylation of proteins involved in the proximal part of the TCR/CD3 transduction pathway.5,6 However, for several years after these alterations were described reasonable mechanistic explanations were lacking. A significant discovery followed: T cells from a high proportion of patients with SLE (approximately 80%) lack a component of the TCR‐associated signalling complex, the CD3ζ chain;7 the defect is found both at the protein and mRNA levels.5,8 Further work has demonstrated that in lupus T cells a surrogate molecule takes the place of the missing CD3ζ chain. A molecule normally absent in T cells, the Fc receptor (FcR)γ chain (originally identified as a component of the FcεRI), is found associated with the TCR/CD3 complex instead of the CD3ζ chain.9 The impostor is fully functional and its presence leads to the establishment of an alternative signalling transduction pathway. It undergoes phosphorylation following CD3ζ cross‐linking and associates with Syk, a kinase normally absent in normal T cells but abundantly expressed in lupus T cells.10 The resultant FcRγ–Syk duet is known to signal approximately 100 times more effectively than the conventional couple ζ–ζ associated protein (ZAP‐70).11 Thus, this rewiring of the lupus TCR, where the FcRγ–Syk complex replaces the ζ–ZAP‐70 complex, explains, at least in part, the aforementioned hypersensitivity to CD3‐mediated stimulation.3,10 The former notion was proven with the demonstration that the artificial expression of FcRγ in normal T cells mimics some of the changes described in lupus T cells, namely the augmented Ca2+ response to TCR stimulation and, interestingly, the decrease in the CD3ζ chain levels.12 Furthermore, treatment of SLE‐derived T cells with piceatannol, at a concentration in which it exclusively inhibits Syk (20 μM), led to the correction of the augmented calcium response and to a significant decrease in the presence of several abnormally phosphorylated proteins.10
The search for the mechanism responsible for the downregulation of CD3ζ in lupus T cells has proven to be a complex quest in which several underlying alterations have been found (box 1).13,14,15,16,17,18
Box 1 Mechanisms of TCR ζ chain deficiency in SLE T cells
Unstable TCR ζ chain mRNA – short 3'UTR
Production of alternatively spliced (non‐functional) TCR ζ chain mRNA
Impaired translation of TCR ζ chain mRNA
-
Impaired TCR ζ chain gene transcription
-
-
Decreased binding of the enhancer Elf‐1
-
-
Increased binding of the repressor CREM
-
-
Increased caspase‐3‐mediated proteolysis
Initially, it became evident that decreased translation was involved. However, neither mutations nor polymorphisms have been found in the ζ chain promoter at the level of genomic DNA that could account for the reduced translation levels. Nevertheless, the ability of SLE T cells to drive the expression of a reporter gene carrying the wild‐type promoter is clearly hampered. An alteration in the post‐translational mechanisms that lead to the production of functional Elf‐1 (E‐74‐like factor), has been found to be responsible for such a defect. Patients with SLE exhibit decreased production of the 98‐KDa DNA binding form of this transcription factor.14
Further work has revealed that other abnormalities, particularly a conspicuous increase in the splice variation of the ζ chain mRNA, contributes to its decreased expression. Several splicing abnormalities have been found including splice insertion and splice deletion variants;19 the latter result from deletions of one or more exons. Reverse transcriptase (RT) PCR analysis has also revealed the existence of a novel ζ chain transcript with an alternatively spliced 3′‐UTR region that is generated by the deletion of nucleotides from 672 to 1233 of ζ chain mRNA. This novel transcript is scarce in normal T cells but represents the dominant form of mRNA in SLE T cells. The ζ chain mRNA with alternatively spliced 3′‐UTR region is less stable, and its presence leads to reduced expression of CD3ζ .8,13
The mechanisms that decrease CD3ζ chain expression in SLE T cells are not limited, however, to decreased transcription and dysfunctional splicing. An additional problem appears to play a role: the half‐life of the CD3ζ chain (measured as protein levels) is reduced.16 Several processes could account for such a phenomenon, but caspase‐3‐mediated proteolysis seems to be primarily involved. SLE‐derived T cells have increased expression and activity of caspase‐3, and CD3ζ bears several caspase‐cleaving sites in its cytoplasmic domain.20,21 Treatment of T cells from patients with lupus with DEVD (a caspase‐3 inhibitor) increases the cellular levels of the ζ chain without altering the expression of other closely related molecules (such as CD3ζ). Interestingly, the same treatment does not cause any change in ζ chain levels in normal T cells (or in T cells from patients with Sjögren's syndrome), which suggests that caspase‐3‐mediated proteolysis does not regulate ζ chain levels in normal T cells.16
The reduction of the ζ chain levels, along with the reciprocal increase in the expression of FcRγ, is directly responsible for some of the phenotypic abnormalities characteristic of lupus T cells.22 Accordingly, replenishment of the ζ chain leads to the downregulation of the FcRγ chain and subsequently to the correction of the TCR/CD3‐induced increased calcium response and the abnormal phosphorylation of cellular substrates. Furthermore, it increases the production of IL2 (see below).23 The former can be also achieved by the inhibition of caspase‐3 activity.16
Pre‐clustered lipid rafts
The molecules that mediate signal transduction are distributed in a non‐random fashion throughout the cell membrane; they are enriched within sections with high levels of cholesterol and gangliosides. These zones have been called lipid rafts and their function is to facilitate and coordinate close interactions between critical molecules in order to facilitate the amplification of downstream signalling events. In normal T cells, TCR ligation induces a rapid clustering of lipid rafts that leads to the concentration of signalling proteins at the immunological synapse.24,25
Membrane morphology and composition are altered in lupus T cells. They possess a larger quantity of lipid rafts, both in resting cells and after stimulation. Moreover, lipid rafts are already clustered in a large fraction of the lupus T cells despite the absence of an obvious stimulus.26,27,28 The aforementioned observation is congruent with the behaviour of SLE‐derived T cells upon TCR‐mediated stimulation. CD3 and LAT, among other molecules involved in signal transduction, are known to localise and to signal through lipid rafts.29,30 Thus, the pre‐clustered rafts could allow faster responses of a higher magnitude to occur. Further, the CD3ζ chain is known to be associated with lipid rafts, and in fact in SLE T cells, even though the ζ chain is quantitatively diminished, the remaining fraction is predominantly localised within the membrane fractions that correspond to lipid rafts.27 In addition, the molecular composition of the lipid rafts from T cells derived from patients with SLE shows several abnormalities. Apart from CD3ζ and LAT (which are expected elements), FcRγ, active Syk kinase and PLCγ1 are present.26 These alterations have direct functional consequences and are probably directly linked to the heightened calcium response observed in SLE T cells.
Interestingly, some of the molecular abnormalities described in the preceding section, namely the decrease in the CD3ζ chain and the increased caspase‐3 activity, have been shown to be related to the pre‐clustered lipid raft phenotype present in SLE T cells. Thus, the replenishment of the ζ chain has been shown to diminish the pre‐clustering of lipid rafts in T cells from patients with SLE; blocking caspase‐3 activity with specific inhibitors has a similar effect.16
Increased migration capacity
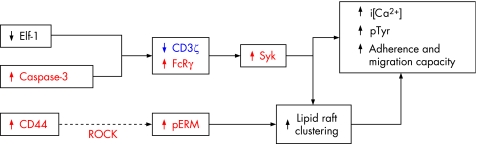
The abnormal signalling pathways used by SLE T cells as well as the pre‐clustered lipid rafts that allow faster and greater signal amplification grant an increased inflammatory capacity to the lupus T cell. Accordingly, the altered lipid raft composition outlined above is associated with an increase in the speed of actin polymerisation that follows CD3‐mediated stimulation.10 Further, SLE T cells display an increased ability to adhere and migrate in response to chemotactic factors than T cells obtained from healthy individuals or from patients with rheumatoid arthritis.31 CD44 is a surface molecule that has been shown to participate in T cell adhesion and migration. It signals through a group of proteins (ezrin, radixin and moesin; ERM) that become phosphorylated on threonine residues and convey a signal that leads to formation of the uropod.32
T cells from patients with SLE exhibit increased expression of CD44 and a parallel increase in the phosphorylation of ERM proteins (pERM). Furthermore, CD44, pERM and F‐actin are distributed in a polar fashion in SLE T cells, forming caps that are normally only observed following cell stimulation. Such polar caps are indeed pre‐clustered lipid rafts, and their presence depends on the phosphorylation of ERM proteins and the integrity of the cytoskeleton. Hence, treatment of SLE T cells with Y27632, a specific inhibitor of Rho kinase (the enzyme that phosphorylates ERM), disrupts the formation of polar caps and hampers the increased adhesion capacity characteristic of SLE T cells. The addition of cytochalasin D (an actin polymerisation inhibitor) or CD44 knockdown (by siRNA) has comparable effects.31 The importance of the former findings was highlighted by the demonstration that in kidney biopsies from patients with lupus glomerulonephritis, infiltrating T cells expressed CD44 and pERM. In contrast, biopsies from allografts undergoing rejection displayed CD44+ T cells, but lacked pERM expression.31
Decreased IL2 production
IL2 has always been a puzzling cytokine. For years after its initial discovery it was regarded as a T cell‐derived autocrine growth factor. It is considered an essential component of the T cell activation process. Its transcription begins soon after the T cell receives a productive stimulus and the T cell relies heavily on its presence in order to proliferate and develop effector functions. Nevertheless, evidence has shown that it plays a key role in the immune regulation process, such that its absence leads to autoimmunity and not to immune deficiency as predicted earlier.33 Interestingly, a phenotypic hallmark of the lupus T cell is a failure to produce normal amounts of IL2 upon activation.34 Such deficiency can directly contribute to several of the disease characteristics such as decreased T cell responses, defective activation‐induced cell death, and altered regulatory T cell homeostasis and function.35,36,37
IL2 production is primarily controlled at the transcriptional level.38 Sites for several transcriptional factors (NF‐κB, AP‐1, NF‐AT, CREB/CREM) have been described in the IL2 promoter, and optimal IL2 levels are only achieved when all the sites are occupied.39 The occupation of several of the promoter sites is abnormal in T cells from patients with SLE. NF‐κB nuclear activity has been shown to be diminished, and the artificial replenishment of the p65 subunit increases IL2 production in these cells.40,41
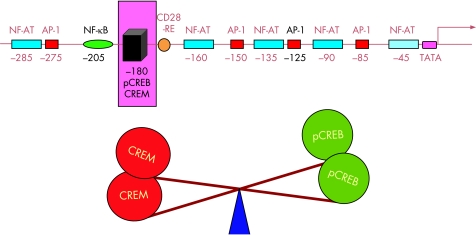
Another position that has been shown to be an abnormally regulated site in SLE T cells is the –180 position. It comprises a binding site for CREB/CREM. When CREB is phosphorylated (pCREB), it acts as a positive factor, greatly enhancing transcription. On the other hand, CREM displaces pCREB and thus acts as a transcriptional repressor.42 It has been proposed that the balance between these two factors is extremely important for the regulation of IL2 transcription.43
A consistent finding in the IL2 promoter of T cells obtained from patients with lupus has been an imbalance in the CREM/CREB ratio found at the –180 site. It represents the fact that T cells from patients with lupus exhibit abnormally high levels of the transcriptional repressor CREM. This abnormality has been directly linked to the characteristic IL2 production defect of lupus T cells.44 In fact, it is probably the final common pathway for several of the defects that have been associated with the IL2 production defect of the lupus T cell (fig 1).
Figure 1 The –180 site in the IL2 promoter is a key regulatory site. Binding sites for several transcription factors have been identified in the IL2 promoter. However, an imbalance of pCREB and CREM in the occupation of the –180 site plays a highly relevant role in the IL2 production defect characteristic of SLE T cells.
CREM inactivation by an antisense CREM plasmid is capable of correcting the abnormally increased CREM binding present in lupus T cells and, by doing so, it directly mitigates the hampered IL2 secretion.45
Numerous factors have been detected in T cells derived from patients with SLE that directly or indirectly affect the balance between CREB and CREM. Interestingly, some of these aberrations appear to be intrinsic to the T cell, whereas others reside in the sera of lupus patients. A mechanism by which SLE serum is able to inhibit IL2 transcription is found in its capacity of activating a kinase called Ca2+/calmodulin‐dependent kinase IV (CaMKIV).46 We have reported evident alterations in the levels and intracellular compartmentalisation of this kinase. Lupus T cells have more CaMKIV and it has been demonstrated that such alteration is a direct consequence of factors present in the sera of patients with lupus, particularly those in which anti‐CD3/TCR activity is detected.46 By a still unknown mechanism, lupus‐derived sera produces migration of CaMKIV into the nuclei of normal T cells. In the nucleus, this kinase is able to activate CREM and increase its binding to the –180 site. This leads to a reduced pCREB/CREM ratio and to a reduction in IL2 transcription. As expected, lupus T cells exhibit increased amounts of CaMKIV in the nuclei. The importance of this phenomenon is highlighted by the fact that inhibition of CaMKIV activity by overexpression of a dominant negative CaMKIV isoform abolishes the IL2 inhibiting effect of SLE sera.46
As expected, CREB and CREM are involved in the regulation of other genes and changes in their levels alter the expression of several proteins apart from IL2; c‐fos, which assembles with jun to form AP‐1, is one of the genes whose transcription is hampered by excessive amounts of CREM. Thus, although alterations of the CREB/CREM ratio probably lead to skewed transcription of a large number of genes, IL2 transcription is affected by at least two distinct mechanisms: the altered occupation of the –180 site, and decreased AP‐1 site occupation (fig 1).45
PP2A (protein phosphatase 2A) is a highly conserved enzyme present in virtually all eukaryotic cells.47 It is the principal enzyme responsible for the dephosphorylation (and thus inactivation) of CREB in T lymphocytes, and has been shown to be able to suppress IL2 production.48,49 PP2A levels, measured as protein and mRNA, are abnormally elevated in T cells from patients with SLE.50 PP2A activity is also augmented in lupus T cells and contributes to the defect in IL2 production by altering the pCREB/CREM ratio. Inhibition of PP2A in lupus T cells (by siRNA or by the expression of dominant negative isoforms) increases pCREB binding to the promoters of c‐fos and IL2 and, by doing so, corrects the IL2 production defect (fig 2).50
Figure 2 Abnormally increased expression of the phosphatase PP2A alters IL2 production in SLE T cells. The increased activity of PP2A limits transcription by two different mechanisms. It directly diminishes CREB phosphorylation and binding to the IL2 promoter, and it decreases AP‐1 formation by altering c‐fos production.
Thus, T cells from patients with SLE have a number of alterations which, through different mechanisms, result in limited ability to produce IL2. Some of the defects result in increased CREM levels, whereas others result in decreased CREB phosphorylation and thus its capacity to stimulate gene transcription.51
Molecular targets
According to what has been outlined before, several molecules have arisen in the last few years that represent plausible therapeutic targets (figs 3, 4). The studies that have been described predict that the inhibition or replenishment of certain pivotal molecules will lead to the correction of the bizarre behaviour that the lupus T cells exhibit during in vitro analyses.
Figure 3 Several defects contribute to the lipid raft pre‐clustering and abnormal T cell activation process in SLE T cells. Many of the molecules involved represent molecular targets; the suppression of those shown in red and the replenishment of the ones depicted in blue associate with the correction of the lupus phenotype.
Figure 4 T cell intrinsic defects as well as factors present in lupus sera contribute to the defective IL2 production characteristic of SLE T cells. The expression of NF‐κB subunits, as well as the inhibition of PP2A and CREM, lead to the correction of the IL2 production defect in T cells obtained from patients with lupus.
The pathological mechanisms that have been described imply that the phenotypic T cell defects are susceptible to being corrected by the alteration of the expression levels and function of more than one molecule. Thus, the correction of the aberrant T cell activation process is theoretically possible by the inhibition of caspase‐3, CD44, Rho kinase (ROCK), FcRγ and Syk; the former would have similar effects to achieving an increased expression of CD3ζ chain (fig 3). Some of these interventions would probably improve the IL2 production defect, but the inhibition of PP2A and CREM would almost certainly accomplish it (fig 4).
As indicated before, the prevalence of the described defects is not homogeneous within the population of patients with SLE. Thus, each individual patient will probably exhibit a distinct combination of abnormalities that will define which interventions are more adequate in that particular case. Furthermore, it will be necessary to identify the relationship between the different phenotypic aberrations and discrete clinical syndromes. A straightforward liaison is predicted to exist between increased expression of CD44, increased phosphorylation of ERM, and T cell infiltration and subsequent kidney inflammation.31
Obviously, an important issue is whether the correction of these deficiencies will translate into clinical improvement. The experimental data strongly suggest that targeting of these abnormally expressed molecules will result in improvement of the involved pathogenic mechanisms which in turn will prove to be of great clinical importance. This concept, however, is yet to be proven and its significance will have to be considered within clinical practice.
Footnotes
Funding: The work summarised in this article has been supported by DHHS, NIH grants R01AI42269, RO1AI49954 and R01AI068787.
Competing interests: None declared.
References
- 1.Parijs L V, Abbas A K. Homeostasis and self‐tolerance in the immune system: turning lymphocytes off. Science 1998280243–248. [DOI] [PubMed] [Google Scholar]
- 2.Kyttaris V C, Juang Y T, Tsokos G C. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol 200517518–522. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos G C, Nambiar M P, Tenbrock K, Juang Y T. Rewiring the T‐cell: signaling defects and novel prospects for the treatment of SLE. Trends Immunol 200324259–263. [DOI] [PubMed] [Google Scholar]
- 4.Vassilopoulos D, Kovacs B, Tsokos G C. TCR/CD3 complex‐mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J Immunol 19951552269–2281. [PubMed] [Google Scholar]
- 5.Liossis S N, Ding X Z, Dennis G J, Tsokos G C. Altered pattern of TCR/CD3‐mediated protein‐tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest 19981011448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsokos G C, Liossis S N. Immune cell signaling defects in lupus: activation, anergy and death. Immunol Today 199920119–124. [DOI] [PubMed] [Google Scholar]
- 7.Nambiar M P, Mitchell J P, Ceruti R P, Malloy M A, Tsokos G C. Prevalence of T cell receptor zeta chain deficiency in systemic lupus erythematosus. Lupus 20031246–51. [DOI] [PubMed] [Google Scholar]
- 8.Nambiar M P, Enyedy E J, Warke V G, Krishnan S, Dennis G, Wong H K.et al T cell signaling abnormalities in systemic lupus erythematosus are associated with increased mutations/polymorphisms and splice variants of T cell receptor zeta chain messenger RNA. Arthritis Rheum 2001441336–1350. [DOI] [PubMed] [Google Scholar]
- 9.Enyedy E J, Nambiar M P, Liossis S N, Dennis G, Kammer G M, Tsokos G C. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum 2001441114–1121. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan S, Chowdhury B, Fisher C U, Nguyen H T, Nambiar M P, Kyttaris V.et al Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. In revision [DOI] [PMC free article] [PubMed]
- 11.Nadler M J S, Mathews S A, Turner T, Kinet J P. Signal transduction by the high‐affinity immunoglobulin E receptor FcRI: coupling form to function. Adv Immunol 200076325–355. [DOI] [PubMed] [Google Scholar]
- 12.Nambiar M P, Fisher C U, Kumar A, Tsokos C G, Warke V G, Tsokos G C. Forced expression of the Fc receptor gamma‐chain renders human T cells hyperresponsive to TCR/CD3 stimulation. J Immunol 20031702871–2876. [DOI] [PubMed] [Google Scholar]
- 13.Nambiar M P, Enyedy E J, Warke V G, Krishnan S, Dennis G, Kammer G M.et al Polymorphisms/mutations of TCR‐zeta‐chain promoter and 3′ untranslated region and selective expression of TCR zeta‐chain with an alternatively spliced 3′ untranslated region in patients with systemic lupus erythematosus. J Autoimmun 200116133–142. [DOI] [PubMed] [Google Scholar]
- 14.Juang Y T, Tenbrock K, Nambiar M P, Gourley M F, Tsokos G C. Defective production of functional 98‐kDa form of Elf‐1 is responsible for the decreased expression of TCR zeta‐chain in patients with systemic lupus erythematosus. J Immunol 20021696048–6055. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury B, Tsokos C G, Krishnan S, Robertson J, Fisher C U, Warke R G.et al Decreased stability and translation of T cell receptor zeta mRNA with an alternatively spliced 3′‐untranslated region contribute to zeta chain down‐regulation in patients with systemic lupus erythematosus. J Biol Chem 200528018959–18966. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Kiang J G, Fisher C U, Nambiar M P, Nguyen H T, Kyttaris V C.et al Increased caspase‐3 expression and activity contribute to reduced CD3zeta expression in systemic lupus erythematosus T cells. J Immunol 20051753417–3423. [DOI] [PubMed] [Google Scholar]
- 17.Tenbrock K, Kyttaris V C, Ahlmann M, Ehrchen J M, Tolnay M, Melkonyan H.et al The cyclic AMP response element modulator regulates transcription of the TCR zeta‐chain. J Immunol 20051755975–5980. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury B, Krishnan S, Tsokos C G, Robertson J W, Fisher C U, Nambiar M P.et al Stability and translation of TCR zeta mRNA are regulated by the adenosine‐uridine‐rich elements in splice‐deleted 3′ untranslated region of zeta‐chain. J Immunol 20061778248–8257. [DOI] [PubMed] [Google Scholar]
- 19.Tsuzaka K, Nozaki K, Kumazawa C, Shiraishi K, Setoyama Y, Yoshimoto K.et al TCRzeta mRNA splice variant forms observed in the peripheral blood T cells from systemic lupus erythematosus patients. Springer Semin Immunopathol 200628185–193. [DOI] [PubMed] [Google Scholar]
- 20.Xue C, Lan‐Lan W, Bei C, Jie C, Wei‐Hua F. Abnormal Fas/FasL and caspase‐3‐mediated apoptotic signaling pathways of T lymphocyte subset in patients with systemic lupus erythematosus. Cell Immunol 2006239121–128. [DOI] [PubMed] [Google Scholar]
- 21.Gastman B R, Johnson D E, Whiteside T L, Rabinowich H. Caspase‐mediated degradation of T‐cell receptor z‐chain. Cancer Res 1999591422–1427. [PubMed] [Google Scholar]
- 22.Tsuzaka K, Setoyama Y, Yoshimoto K, Shiraishi K, Suzuki K, Abe T.et al A splice variant of the TCR zeta mRNA lacking exon 7 leads to the down‐regulation of TCR zeta, the TCR/CD3 complex, and IL‐2 production in systemic lupus erythematosus T cells. J Immunol 20051743518–3525. [DOI] [PubMed] [Google Scholar]
- 23.Nambiar M P, Fisher C U, Warke V G, Krishnan S, Mitchell J P, Delaney N.et al Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3‐induced interleukin‐2 production in patients with systemic lupus erythematosus. Arthritis Rheum 2003481948–1955. [DOI] [PubMed] [Google Scholar]
- 24.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997387569–572. [DOI] [PubMed] [Google Scholar]
- 25.Brown D A, London E. Structure and function of sphingolipid‐ and cholesterol‐rich membrane rafts. J Biol Chem 200027517221–17224. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan S, Nambiar M P, Warke V G, Fisher C U, Mitchell J, Delaney N.et al Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol 20041727821–7831. [DOI] [PubMed] [Google Scholar]
- 27.Nambiar M P, Enyedy E J, Fisher C U, Krishnan S, Warke V G, Gilliland W R.et al Abnormal expression of various molecular forms and distribution of T cell receptor zeta chain in patients with systemic lupus erythematosus. Arthritis Rheum 200246163–174. [DOI] [PubMed] [Google Scholar]
- 28.Jury E C, Kabouridis P S, Flores‐Borja F, Mageed R A, Isenberg D A. Altered lipid raft‐associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest 20041131176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janes P W, Ley S C, Magee A I, Kabouridis P S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 20001223–34. [DOI] [PubMed] [Google Scholar]
- 30.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity 19988723–732. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Harada T, Juang Y T, Kyttaris V C, Wang Y, Zidanic M.et al Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol 20071781938–1947. [DOI] [PubMed] [Google Scholar]
- 32.del Pozo M A, Nieto M, Serrador M, Sancho D, Vicente‐Manzanares M, Martinez C.et al The two poles of the lymphocyte: specialized cell compartments for migration and recruitment. Cell Adhes Commun 19986125–133. [DOI] [PubMed] [Google Scholar]
- 33.Ma A, Koka R, Burkett P. Diverse functions of IL‐2, IL‐15, and IL‐7 in lymphoid homeostasis. Annu Rev Immunol 200624657–679. [DOI] [PubMed] [Google Scholar]
- 34.Alcocer‐Varela J, Alarcon‐Segovia D. Decreased production of and response to interleukin‐2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest 1982691388–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs B, Vassilopoulos D, Vogelgesang S A, Tsokos G C. Defective CD3‐mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF‐alpha. Clin Immunol Immunopathol 199681293–302. [DOI] [PubMed] [Google Scholar]
- 36.Crispin J C, Martinez A, Alcocer‐Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 200321273–276. [DOI] [PubMed] [Google Scholar]
- 37.Valencia X, Yarboro C, Illei G, Lipsky P E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 20071782579–2588. [DOI] [PubMed] [Google Scholar]
- 38.Rothenberg E V, Ward S B. A dynamic assembly of diverse transcription factors integrates activation and cell‐type information for interleukin 2 gene regulation. PNAS 1996939358–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H P, Imbert J, Leonard W J. Both integrated and differential regulation of components of the IL‐2/IL‐2 receptor system. Cytokine Growth Factor Rev 200617349–366. [DOI] [PubMed] [Google Scholar]
- 40.Wong H K, Kammer G M, Dennis G, Tsokos G C. Abnormal NF‐kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65‐RelA protein expression. J Immunol 19991631682–1689. [PubMed] [Google Scholar]
- 41.Herndon T M, Juang Y T, Solomou E E, Rothwell S W, Gourley M F, Tsokos G C. Direct transfer of p65 into T lymphocytes from systemic lupus erythematosus patients leads to increased levels of interleukin‐2 promoter activity. Clin Immunol 2002103145–153. [DOI] [PubMed] [Google Scholar]
- 42.Tenbrock K, Tsokos G C. Transcriptional regulation of interleukin 2 in SLE T cells. Int Rev Immunol 200423333–345. [DOI] [PubMed] [Google Scholar]
- 43.Tenbrock K, Juang Y T, Tolnay M, Tsokos G C. The cyclic adenosine 5′‐monophosphate response element modulator suppresses IL‐2 production in stimulated T cells by a chromatin‐dependent mechanism. J Immunol 20031702971–2976. [DOI] [PubMed] [Google Scholar]
- 44.Tenbrock K, Juang Y T, Gourley M F, Nambiar M P, Tsokos G C. Antisense cyclic adenosine 5′‐monophosphate response element modulator up‐regulates IL‐2 in T cells from patients with systemic lupus erythematosus. J Immunol 20021694147–4152. [DOI] [PubMed] [Google Scholar]
- 45.Kyttaris V C, Juang Y T, Tenbrock K, Weinstein A, Tsokos G C. Cyclic adenosine 5′‐monophosphate response element modulator is responsible for the decreased expression of c‐fos and activator protein‐1 binding in T cells from patients with systemic lupus erythematosus. J Immunol 20041733557–3563. [DOI] [PubMed] [Google Scholar]
- 46.Juang Y T, Wang Y, Solomou E E, Li Y, Mawrin C, Tenbrock K.et al Systemic lupus erythematosus serum IgG increases CREM binding to the IL‐2 promoter and suppresses IL‐2 production through CaMKIV. J Clin Invest 2005115996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 2001353417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadzinski B E, Wheat W H, Jaspers S, Peruski L F, Lickteig R L, Johnson G L.et al Nuclear protein phosphatase 2A dephosphorylates protein kinase A‐phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol 1993132822–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kracht M, Heiner A, Resch K, Szamel M. Interleukin‐1‐induced signaling in T‐cells. Evidence for the involvement of phosphatases PP1 and PP2A in regulating protein kinase C‐mediated protein phosphorylation and interleukin‐2 synthesis. J Biol Chem 199326821066–21072. [PubMed] [Google Scholar]
- 50.Katsiari C G, Kyttaris V C, Juang Y T, Tsokos G C. Protein phosphatase 2A is a negative regulator of IL‐2 production in patients with systemic lupus erythematosus. J Clin Invest 20051153193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katsiari C G, Tsokos G C. Transcriptional repression of interleukin‐2 in human systemic lupus erythematosus. Autoimmun Rev 20065118–121. [DOI] [PubMed] [Google Scholar]