Abstract
In this manuscript we discuss the reasons why and how health economics is important, the type of economic studies that are relevant in healthcare to different stakeholders in general, and what analyses can and have been performed in the field of rheumatoid arthritis (RA). We will thus specifically address costs and outcome measurements in RA, as well as the need for modelling in chronic progressive diseases.
Health economics is the application of the discipline and the methods of economics to the topic “health”. Thus, healthcare is considered to be no different from any other productive sector of the economy: resources are used and investments are made to produce “health”. Health economics is then concerned with achieving efficiency in the allocation of resources in healthcare, a sector where a large part of the expenses are carried by the public budget (around 70–75% in Europe, Canada and Australia, and around 40–45% in the USA; www.oecd.org).
The theory and the methods of health economics apply across the world. However, there is great variation in the amount of resources used for healthcare across countries, due to general economic factors. There will hence be significant variation in access to the newest and most effective treatments. Thus, economic analyses are always country specific, and it is impossible to conclude from the results of one study in a given country on the possible situation in another country. The organisation of care differs, treatment patterns vary, and the relative and absolute cost of individual resources can be very different.
Today, information about new treatments is widely available. Few patients with RA ignore the existence of the biological drugs (TNFα inhibitors). These treatments are highly effective, but also come at a high cost. Their price is global (ie, similar across countries in order to avoid parallel trade), and economic factors will therefore be a significant source of variations in access to these drugs. Acceptability of their cost will be different in the USA with a per capita spending on healthcare of $6000 and Bulgaria with a spending of $600. Within the relationship between patients (consumers), providers (agents) and payers, the latter group is becoming more and more important. All payers are interested in opportunities to do something at a lower cost to make room for other payments (cost containment). They are also concerned about the impact on their budget, that is, the cost of a treatment and the estimated number of treatments (budget impact). However, in particular, public payers are also interested in cost‐effectiveness, asking the question how a given payment will contribute to outcome in terms of survival, quality of life (QoL) and quality of care (value for money). Thus, payers dictate to some extent what treatments can/cannot be used on their budget. This is particularly important in RA, where – without health insurance, be it public or private – few patients will be able to afford the anti‐TNF drugs.
Payers, while still demanding innovative treatments, will no longer be impressed with innovative technology, unless it truly improves the outcomes for patients. One could argue that this is clearly the case for the anti‐TNF drugs – yet, many restrictions apply to their usage, due to affordability issues. Cost‐effectiveness has thus become an important additional criterion for selecting how to use healthcare resources most efficiently, using economic evaluation as the tool. These studies will provide data in a structured format, but will not make an explicit decision concerning the value of the benefit nor the acceptability of its cost.
Types of economic studies
In health care, there are basically two types of economic analyses (table 1):
Table 1 Types of economic analyses.
| Type of analysis | Effectiveness measure | Use |
|---|---|---|
| Descriptive studies | ||
| Cost‐of‐ illness study | None | Description of all costs related to a disease. Policy information and basis for economic evaluation |
| Economic evaluations | ||
| Cost‐effectiveness analyses | ||
| Cost minimisation | Not measured, as it assumes that the effect of the alternatives is identical | Comparison of treatments within the same indication |
| Cost‐effectiveness | Uni‐dimensional disease‐specific measure (eg, patients cured, life‐years saved, disease‐free time) | Comparison of treatments within the same indication |
| Cost utility | Multidimensional outcome measure combining quality of life and life expectancy | Comparison across indications |
| Cost–benefit analysis | ||
| Cost benefit | Health benefit expressed in monetary terms (eg, willingness to pay) | Comparison across different sectors of the economy |
descriptive studies that simply describe what can be observed (positive theory)
evaluative studies that attempt to estimate what would happen if a change (eg, a new treatment) to what has been observed is introduced (normative theory).
Descriptive studies
Cost‐of‐illness studies observe what costs are caused by and related to a certain disease, within a given timeframe (generally 1 year) and within a specific geographical area (generally a country).
Studies can give very different results,1,2 and table 2 illustrates some of the key sources for differences. Each study must therefore be analysed in detail to interpret the results.
Table 2 Some sources for differences in cost‐of‐illness studies.
| Topic | Differences |
|---|---|
| Perspective of costs | Societal perspective (all costs regardless of who pays, ie, direct and indirect costs) |
| Payer perspective (only costs that are covered by the specific payer, generally only direct costs) | |
| Definition of sample | Population sample (representative of the entire patient population in a geographic area) |
| Specific sample (eg, by disease severity, by age, by point of care) | |
| Definition of relevant costs | Consumption of patients with RA (all consumption of a patient with the disease) |
| Consumption for RA (only RA‐related care and consumption) | |
| Mode of data collection | Top‐down (from national statistical databases) vs bottom‐up (from medical charts and patients) |
| Prospective (following a sample of patients over time) vs retrospective (consumption in the past 1, 3, 12 months) | |
| Valuation | Tariffs (costs fixed by an insurance) |
| Opportunity costs (costs in their next best alternative) |
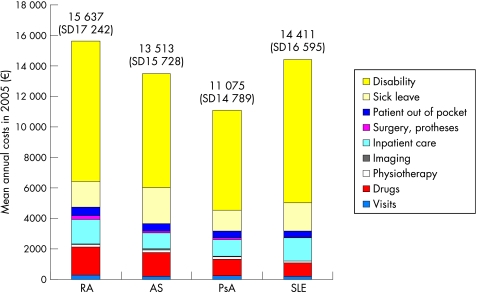
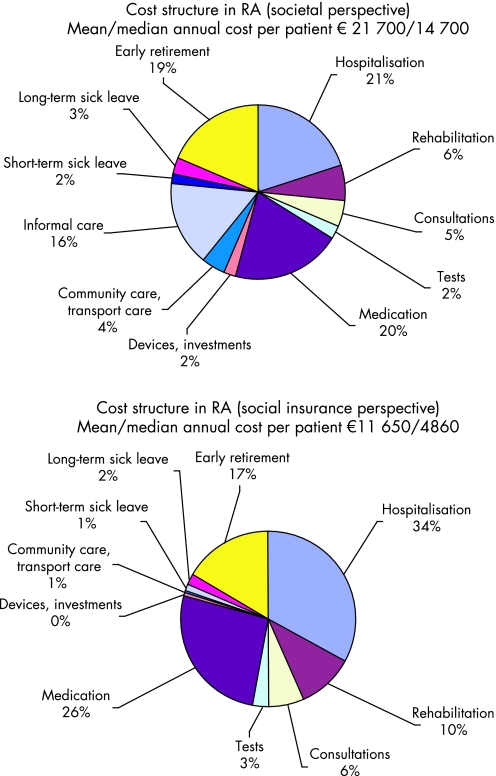
Two recent studies among the wealth of cost‐of‐illness studies in RA will serve to illustrate these issues, one in Germany3 and one in France4 (figs 1, 2). At first glance, the differences are striking: Total annual costs per patient amount to €15 600 in Germany and to €21 700 in France; indirect costs represent over 70% in Germany but only 24% in France. However, with a closer look, these differences are relatively easy to explain.
Figure 1 Cost structure in rheumatic diseases for patients <65 years in Germany (adapted from Huscher et al.3). AS, ankylosing spondylitis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Figure 2 Cost structure for different perspectives in France (€2005).4 The study included 1487 patients with a mean age of 63 and 18 years' disease duration. The mean Health Assessment Questionnaire score was 1.4, mean patient Visual Analogue Scale was 4.4. The sample was slightly biased towards patients treated with anti‐TNF drugs (27% compared to an estimated national 20%).This explains the high proportion of costs represented by the biological treatments. 55% of patients were treated with methotrexate and 28% with other disease‐modifying anti‐rheumatic drugs.
An important point to remember is that the more complete the inclusion of types of costs, the smaller the individual proportions become. For instance, the German study only included part of the costs borne by patients and in particular ignored family help (informal care), while these items amounted to 18–20% in the French study.
The German sample only included patients of working age (<65 years), hence every patient on sick leave or out of the workforce due to RA contributed to indirect costs. Contrary to this, more than half of the patients in France were above the formal retirement age (60), and for these neither sick leave nor loss of work capacity is counted.
The results from the French study also allow us to illustrate the difference in costs between the societal and payer perspectives. Only slightly over half of the total cost of the disease is paid for by the national health and invalidity insurance.
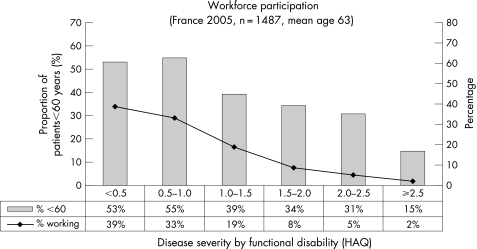
Hence “average costs per patient” are not comparable between studies, unless the sample and the methodology are identical. But regardless of the study, the loss of work capacity has been identified as the largest contributor to total costs, and it is interesting to analyse the effect of disease severity on indirect costs, as shown in fig 3 for the French study. Indeed, workforce participation declines rapidly as the disease progresses. When functional disability is not or is minimally impaired, 60–74% of patients below 60 are employed, which is similar to the normal workforce participation (64% for women and 75% for men in the age group 15–64, www.insee.fr). However, only around 15% of patients below 60 with severe impairment still work. How long a patient will be able to maintain employment will, however, not depend on the disease alone, but also on age, gender, type of work (education) and, last but not least, the country and its policies for dealing with invalidity.
Figure 3 Workforce participation for patients with RA in France showing the proportion of patients employed, compared to patients below 60 years (the official retirement age in France).4 While as many as 74% of patients with a Health Assessment Questionnaire (HAQ) below 0.5 are working, the proportion shrinks to 13% for patients with a HAQ of 2.5 and above.
Economic evaluations
Economic evaluation relates to social choice and attempts to answer the question whether a new treatment is better, equivalent or less efficacious than the current standard treatment. The underlying objective is to maximise social utility (the outcome for the population as a whole), and such studies are therefore most appropriate in settings where healthcare is publicly financed. It is not surprising therefore that most European countries, as well as Australia and Canada, have a formal process and guidelines in place to include cost‐effectiveness studies in their decision process for resource allocation. These guidelines relate to general methodological principles that, however, may require some adaptation depending on the disease and most of all, the available data. In RA, the OMERACT group has attempted to produce RA specific guidelines.5 Unfortunately, these guidelines have ignored some of the key principles set forward in official guidelines. For instance, they recommend a time horizon of 1 year for the analysis, while all national guidelines require long‐term or even lifetime analysis in chronic diseases.
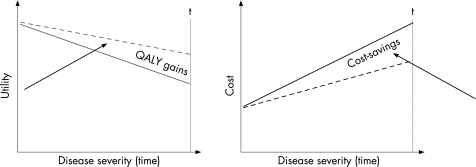
The economic hypothesis in chronic progressive diseases is that costs increase and QoL decreases as the disease worsens over time (fig 4). A treatment that succeeds in changing the speed of progression will change the slopes of these curves and the area between the two curves will represent cost savings and health gains.
Figure 4 Hypothesis for economic evaluation in RA. Cost increase and quality of life (QoL) decreases as the disease worsens (solid lines). If a treatment succeeds in changing the speed of the progression, the slopes of the cost and QoL curves change (dotted lines). The area between the curves will then, over a given timeframe, represent the health gains and cost savings. QALY, quality‐adjusted live year.
Cost‐effectiveness analyses are always comparative and incremental, that is, they report the additional investment required to obtain an additional unit of health benefit with one treatment strategy compared to another strategy. However, to make them useful for decisions on resource allocation, the health benefit must be expressed with a measure that is comparable across diseases. This is not obvious in RA where a number of disease‐specific measures are used to express the effect of the disease and the effectiveness of treatment: swollen and stiff joints, disease activity, joint damage, functional capacity, American College of Rheumatology (ACR) 20/50/70. None of these is directly usable in economic evaluation performed for policy purposes. This is not different from what happens in a number of other diseases. The concept of the quality‐adjusted live year (QALY) has specifically been developed to overcome this problem, as an outcome measure in economic evaluation regardless of the disease analysed. The QALY combines life expectance and QoL by weighing life‐years with a quality index called utility.6 In this framework, utility is defined as the preference that patient and the general population have for given states of health. Utilities are expressed as a value on a scale anchored between 0 (death) and 1 (perfect health). These can be measured directly using techniques from decision analysis (standard gamble, time trade‐off)7,8 or derived from health state systems (“tariffs”) such as those developed for the EQ‐5D9,10 or the Health Utility Index.11 Living 2 years with a utility of 0.5 corresponds to one QALY, which is the same as living 1 year in full health.
In RA, most of the QALY gain stems from improvements in QoL over time. Although the disease clearly increases mortality, the absolute effect on life expectancy is small. An important question is therefore what drives QoL. A number of studies have shown how QoL decreases as functional impairment increases,4,12,13,14,15 making the HAQ a very good disease measure to correlate with utility. However, recently it has been shown that disease activity, most likely through pain, correlates independently from HAQ with utility.15,16 As a consequence, both measures have to be taken into account, in addition to age and gender, when estimating utility.
The same is true for costs, although in a less pronounced manner. Functional capacity has been identified as by far the strongest driver of costs in early studies.12,14 More recently, an additional effect of disease activity on costs has been identified in studies in Sweden.15,16 Short‐term sick leave, which happens most often early in the disease as patients are younger and still in the workforce, was correlated with disease activity, not with function. However, function remained the driver of all other cost types (excluding the cost for anti‐TNF drugs). This can be further illustrated with a study in Germany that correlated costs with disease activity.17 Costs increased from around €600 for patients with low‐disease activity to around €1200 for patients with high‐disease activity. Compared to this, annual costs in a Spanish study increased from very limited costs for patients with no functional impairment to around €25 000 for patients with severe impairment. Similarly, the French study from 2005 mentioned earlier showed a cost increase from around €8000 for patients with a HAQ below 0.5 to around €40 000 for patients with a HAQ of 2.5 or higher.4 In the Swedish study from 2002, costs increased from €5000 to €20 000 for the same levels.
These examples illustrate two points. First, again, costs differ between countries and study results cannot be transferred from one country to another. Even studies with identical methodology can give rather different results in different countries.14 Second, the magnitude of the increase in cost with worsening disease, that is, the slope of the cost curve, will in part drive the results of the cost‐effectiveness results. Indeed, the higher the cost in advanced disease, the more is to be gained by avoiding or delaying these disease states.
Modelling
The need to adopt a long‐term view in RA makes modelling unavoidable. Clinical trials are clearly too short to show the full effect of a treatment that changes the course of a disease and it will take a long time before its effect can be observed in clinical practice. It may in fact never be observable, as new treatments are introduced and patient management modified. Models are a structured representation of an often complicated environment that allow investigating the effect of different hypotheses and scenarios on a number of outcomes, for example, costs and QoL, using the best available information at the time of the analysis.
In chronic diseases models are used to combine different types of data from different sources to overcome the lack of directly observable data. Clinical trial data are directly incorporated, but combined with epidemiological data on long‐term disease progression and mortality, as well as data on costs and utility. Again, this may not be straightforward. Long‐term epidemiological cohorts are often inception cohorts with few of the patients having progressed to very advanced disease. They may be too small to produce reliable estimates of progression. Also, with more potent treatments available, the disease course may have changed since the start of these follow‐ups. More recent databases, such as registries, while often much larger, have a short follow‐up. Part of the disease progression may thus have to be based on average annual progression rather than on actual patient‐level data. Costs and utilities by disease severity are much easier to incorporate, provided obviously that adequate recent data from relatively large and representative samples of patients are available.
Modelling has become the standard methodology for economic evaluation in RA over recent years. Unfortunately, however, as the methodology has developed and computing power increased, models have become more sophisticated and communicating them to a non‐specialist audience has become more difficult.1 Similar modelling studies can give very different results for a number of reasons.1,18,19 Most of the reasons mentioned in table 2 for cost‐of‐illness studies also apply to cost‐effectiveness analyses, but additional factors play an important role. Some of these are mentioned in table 3.
Table 3 Some sources for differences in economic evaluations (models).
| Topic | Differences |
|---|---|
| Outcome measure | Disease‐specific (eg, proportion reaching ACR20) |
| Generic (eg, QALY) | |
| Underlying data | Primary data |
| Summary data from the literature | |
| Perspective | Society or health insurance |
| Definition of sample | Specific clinical trial population |
| General sample from clinical practice | |
| Costs | Tariffs versus opportunity costs |
| Valuation method for indirect costs | |
| Time horizon | Short (within trial) |
| Medium (5–10 years) | |
| Lifetime | |
| Assumptions | Extrapolation beyond the clinical trial |
| Missing information |
QALY, quality‐adjusted life years.
Conclusion
Cost‐effectiveness analyses have become an important tool in decision making in healthcare. Compared to other diseases, such studies have a long tradition in RA. One of the first cost‐effectiveness analyses was carried out as early as 1988.20 The study was a simple within‐trial analysis of a 6‐month clinical study. Not surprisingly, within such a short timeframe, the study found no difference between the two arms. Today, long‐term models based on the risk of disease progression and changes to that risk have become the standard.21,22,23,24,25,26,27,28
Modelling techniques are well developed and relatively standard. Models are, however, only as good as the underlying data and assumptions. Hence, without going into technical details, the non‐specialist reader can carefully analyse a study to understand the relevance of the decision problem that the study attempts to solve, the quality of the data used and the plausibility of the assumptions made.
Abbreviations
QALY - quality‐adjusted live year
QoL - quality of life
RA - rheumatoid arthritis
Footnotes
Competing interests: None declared.
References
- 1.Kobelt G. Health economic issues in Rheumatoid Arthritis. Scand J Rheumatol 200635415–425. [DOI] [PubMed] [Google Scholar]
- 2.Rosery H, Bergemann R, Maxion‐Bergemann S. International variation in resource utilisation and treatment costs for rheumatoid arthritis. A systematic literature review. Pharmacoeconomics 200523243–257. [DOI] [PubMed] [Google Scholar]
- 3.Huscher D, Merkesdal S, Thiele K, Zeidler H, Schneider M, Zink A.et al Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 2006651175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobelt G, Richard B, Peeters P, Sany J. Costs and quality of life of patients with RA in France. ACR abstract 2006; Bone Joint Spine. In press. [DOI] [PubMed]
- 5.Maetzel A, Tugwell P, Boers M, for the OMERACT group Economic evaluation of programs or interventions in the management of rheumatoid arthritis: defining a reference case. J Rheumatol 200330891–896. [PubMed] [Google Scholar]
- 6.Torrance G. Measurement of health state utilities for economic appraisal. A review. J Health Econ 198651–30. [DOI] [PubMed] [Google Scholar]
- 7.Drummond M, O'Brien B, Stoddart G, Torrance G.Methods for the economic evaluation of health care. Boston: Kluwer Academic Publishers, 1997
- 8.Kobelt G.Health economics: introduction to economic evaluation. London: Office of Health Economics, 1996
- 9.The EuroQol Group EuroQol: a new facility for the measurement of health‐related quality of life. Health Policy 199016199–208. [DOI] [PubMed] [Google Scholar]
- 10.Dolan P, Gudex C, Kind P, Williams A.A social tariff for EuroQol: Results from a UK general population survey. Discussion Paper 138. York: Centre for Health Economics, University of York, 1995
- 11.Torrance G.Multi‐attribute value and utility functions for a comprehensive health status classification system. Toronto: McMasters University, 1992
- 12.Kobelt G, Eberhardt K, Jönsson L, Jönsson B. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 199942347–356. [DOI] [PubMed] [Google Scholar]
- 13.Hurst N, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health‐related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ‐5D). Br J Rheumatol 199736551–559. [DOI] [PubMed] [Google Scholar]
- 14.Kobelt G, Jönsson L, Lindgren P, Young A, Eberhardt K. Modelling the progression of rheumatoid arthritis. a two‐country model to estimate costs and consequences of RA. Arthritis Rheum 2002462310–2319. [DOI] [PubMed] [Google Scholar]
- 15.Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology (Oxford) 2005441169–1175. [DOI] [PubMed] [Google Scholar]
- 16.Kobelt G, Eberhardt K, Geborek P. TNF‐inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow‐up study of patients with RA treated with etanercept or infliximab in southern Sweden. Ann Rheum Dis 2004634–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulsemann J L, Ruof J, Zeidler H, Mittendorf T. Costs in rheumatology: results and lessons learned from the ‘Hannover Costing Study'. Rheumatol Int 200526704–711. [DOI] [PubMed] [Google Scholar]
- 18.Drummond M F, Barbieri M, Wong J B. Analytic choices in economic models of treatments for rheumatoid arthritis: what makes a difference? Med Decis Making 200525520–533. [DOI] [PubMed] [Google Scholar]
- 19.Bansback N J, Regier D A, Ara R, Brennan A, Shojania K, Esdaile J M.et al An overview of economic evaluations for drugs used in rheumatoid arthritis: focus on tumour necrosis factor‐alpha antagonists. Drugs 200565473–496. [DOI] [PubMed] [Google Scholar]
- 20.Thompson M, Leighton‐Read J, Hutchings C, Paterson M E H., Jr The cost effectiveness of auranofin. Results of a randomized clinical trial. J Rheumatol 19881535–42. [PubMed] [Google Scholar]
- 21.Kobelt G, Lindgren P, Young A, Eberhardt K. Cost and effects of leflunomide in the UK. Eur J Health Econom 20023173–179. [DOI] [PubMed] [Google Scholar]
- 22.Kobelt G, Jönsson L, Young A, Eberhardt K. The cost‐effectiveness of infliximab (Remicade(R)) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 200342326–335. [DOI] [PubMed] [Google Scholar]
- 23.Wong J, Singh G, Kavanough A. Estimating the cost‐effectiveness of infliximab for rheumatoid arthritis. Am J Med 2002113400–408. [DOI] [PubMed] [Google Scholar]
- 24.Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with chronic conditions: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technology Assessment 200481–91. [DOI] [PubMed] [Google Scholar]
- 25.Brennan A, Bansback N, Reynolds A, Conway P. Modeling the cost‐effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology 20044362–72. [DOI] [PubMed] [Google Scholar]
- 26.Bansback N J, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 200564995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept (Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis 2005641174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K.et al Modelling the cost effectiveness of TNF‐{alpha} antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology (Oxford) 2007461345–1354. [DOI] [PubMed] [Google Scholar]