Abstract
Generation and negative selection of NK1.1+α/β T cell receptor (TCR)+ thymocytes were analyzed using TCR-transgenic (B10.D2 × DO10)F1 and (C57BL/6 × DO10)F1 mice and Rag-1−/−/DO10 mice, which had been established by breeding and backcrossing between Rag-1−/− and DO10 mice. Almost all T cells from these mice were shown to bear Vα13/Vβ8.2 that is specific for chicken ovalbumin (cOVA) and restricted to I-Ad. A normal proportion of the NK1.1+ Vα13/Vβ8.2+ thymocytes was generated in these mice. However, the actual cell number of both NK1.1+ and NK1.1− thymocytes in I-Ad/d mice (positive selecting background) was larger than that in I-Ab/d mice (negative selecting background). Markedly low but significant proportions of NK1.1+ Vα13/Vβ8.2+ cells were detected in the spleens from I-Ad/d and I-Ab/d mice. It was shown that the splenic NK1.1+ T cells of the I-Ab/d mice were anergized against stimulation through TCR. When (B10.D2 × DO10)F1 and (C57BL/6 × DO10)F1 mice were given cOVA, extensive or intermediate elimination of NK1.1+α/βTCR+ thymocytes was induced in I-Ad/d or I-Ab/d mice, respectively. However, the clonal elimination was not as complete as that seen in the major NK1.1− thymocyte population. The present findings indicate that normal generation of NK1.1+α/βTCR+ thymocytes occurs in the absence of Vα14-Jα281 and that substantial negative selection operates on the NK1.1+α/βTCR+ cells.
Keywords: thymus/natural killer T cell/T cell antigen receptor transgenic mouse/negative selection
Mouse NK1.1+α/βTCR+ thymocytes constitute a unique subset among CD4−8− double negative and CD4+8− single positive populations (1–3). This small population of thymocytes expresses low levels of extremely deviated α and β chains of TCR (3, 4). The deviated TCR expression includes in some cases a self-antigen (Ag)-reactive repertoire, which suggests that the NK1.1+α/βTCR+ thymocyte population has undergone an intrathymic selection that is different from that of the major NK1.1− thymocyte population (3, 5). Indeed, it has been shown that the NK1.1+α/βTCR+ thymocytes are not positively selected under the influence of thymic epithelial cells but are generated in the presence of CD4+8+ double positive thymocytes, which express nonpolymorphic major histocompatibility complex class I-like molecules, CD1 (2, 6–9). This is so even though we recently have found that an intact microenvironment of the thymus makes up a component that is also important in generation of the NK1.1+α/βTCR+ thymocytes (10). In addition, Taniguchi et al. (11) and Bendelac et al. (12) reported that expression of an invariant TCR α chain, Vα14-Jα281, is an essential requirement for development of NK1.1+α/βTCR+ cells and biases the differentiation of major NK1.1− thymocyte population toward the NK1.1+ developmental pathway.
Recently, Schulz et al. (13) reported normal development of NK1.1+ Vα3+Vβ8.2+ thymocytes in anti-H-Y (TCR)/Rag-2−/− transgenic mice (Tgm). This report suggested that the expression of Vα14-Jα281 is not necessarily an essential requisite for generation of NK1.1+α/βTCR+ thymocytes. It seemed as though the NK1.1+ Vα3+Vβ8.2+ cells did not undergo negative selection (13, 14). However, it has not been determined precisely whether the NK1.1+α/βTCR+ thymocytes bearing major histocompatibility complex class II-restricted TCR can be generated normally. In addition, it has been unclear whether the NK1.1+α/βTCR+ thymocytes undergo strict negative selection or, as mentioned above, whether or not these cells are significantly influenced by the negative selection (13, 14).
In the present study, using various Tgm bearing TCR (Vα13/Vβ8.2) that are specific for cOVA and restricted to I-Ad, generation and negative selection of NK1.1+α/βTCR+ thymocytes and spleen cells under the influence of a positive or negative selecting background were investigated. We show herein that a normal population of NK1.1+ Vα13/Vβ8.2+ thymocytes is generated in these TCR Tgm. The NK1.1+ Vα13/Vβ8.2+ cells in the spleen of mice with a negative selecting background were anergized against stimulation through TCR. In addition, the majority of the NK1.1+α/βTCR+ thymocytes were eliminated when exposed to the specific Ag in vivo.
MATERIALS AND METHODS
Mice.
BALB/c, C57BL/6 (B6), and B10.D2 (D2) mice were purchased from the Shizuoka Laboratory Animal Cooperation (Hamamatsu, Japan). C57BL/6J-Rag-1-deficient mice (Rag-1−/−) were purchased from The Jackson Laboratory. DO11.10 TCR mice (DO10) bearing TCR (Vα13/Vβ8.2) from a hybridoma, DO11.10, that is specific for chicken OVA (cOVA) (323–339) and restricted to I-Ad were kindly provided by Dr. Loh (15) (Nippon Roche Research Center, Japan) and maintained in the animal facility at Hokkaido University. (B6 × DO10)F1 and (D2 × DO10)F1 mice were bred in our animal facility. Rag-1−/− mice were bred with DO10 mice, and offspring were backcrossed to Rag-1−/− mice for several generations before intercrossing to establish Rag-1−/−/DO10 mice as described previously (13). Mice that lack sIg+ and B220+ cells and in which almost 100% of their CD4+ cells are positive for KJ1-26 were selected as Rag-1−/−/DO10. These mice were maintained in specific pathogen-free conditions.
Antibody and Flow Cytometry.
Thymocytes and spleen cells were isolated as described elsewhere (16). Primary monoclonal antibodies used for immunofluorescence staining and flow cytometry were biotinylated (biotin) KJ1-26 (17, 18) (anti-DO11.10 α/β TCR -clonotype), biotin-PK136 (anti-NK1.1), biotin-MR5–2 (anti-TCRVβ 8.1, 8.2), biotin-M1/69 (anti-heat stable Ag), biotin-MEL-14 (anti-CD62L), biotin-IM7 (anti-CD44), biotin-GL3 (anti-γ/δ TCR), fluorescein isothiocyanate (FITC)-53–6.7 (anti-CD8), PE-PK136, PE-RM4–5 (anti-CD4), FITC-RM4–5, and PE-TM-β1 (anti-IL-2Rβ) (PharMingen). Secondary reagents used for biotin primary antibodies were Streptavidin Red 670 (GIBCO/BRL) or Streptavidin-FITC (Biomedia, Foster City, CA). Prior to staining, cells were incubated with 2.4G2 (anti-FcγR) (19) to block nonspecific staining. Propidium iodide red fluorescence dye (Sigma) was added to the cells immediately before analysis. To determine apoptotic cells, Annexin-V-FITC (Annexin-V-Fluos, Boehringer Mannheim) staining was performed according to manufacturer’s protocol. The stained cells were analyzed by FACScan (Becton Dickinson) as described previously (3, 16).
In Vivo Deletion of Immature Thymocytes.
Mice were given by intraperitoneal injection instead of cOVA peptide employed in the original in vivo deletion model (15) 250 μl of 750 μM cOVA protein daily for 3 days, and the next day the thymocytes were obtained from the mice and analyzed. cOVA (grade VII) was purchased from Sigma. Control mice were injected with PBS alone.
Interleukin-4 (IL-4) Production After Administration of Anti-CD3 or KJ1-26 in Vivo.
(B6 × DO10)F1 and (D2 × DO10)F1 mice were injected intravenously with a single dose of anti-CD3 (4 μg) or KJ1-26 (4 μg). After 1.5 h, spleens were removed and single cell suspensions were prepared. The spleen cells were cultured in culture medium for 2 h, and IL-4 production in the culture supernatants was quantitated with a CT.4S cell line as described elsewhere (20).
Reverse Transcription-PCR Analysis.
Total RNA was extracted from thymuses and spleens of B6 or Rag-1−/−/DO10 according to standard procedure (21). Complementary DNA was synthesized from 1.3 μg of RNA using random hexamer and Moloney murine leukemia virus reverse transcriptase (Superscript, GIBCO/BRL) at 37°C for 1 h in the presence of dNTPs and RNase inhibitor, RNasin (Promega). The cDNA transcripts were used as templates in PCR for amplifications of the following gene products with respective primer pairs: Vα13/JαDO, 5′-CAG GAG GGA TCC AGT GCC AGC-3′/5′-TGG CTC TAC AGT GAG TTT GGT-3′ (Dr. Philip Lucas, personal communication); Vα14/Jα281, 5′-TAA GCA CAG CAC GTG CAC AT-3′/5′-CAA TCA GCT GAG TCC CAG CT-3′ (22); and Cα-for/Cα-rev1, 5′-CCT CTG CCT GTT CAC CGA CT-3′/5′-CAG GAG GAT TCG GAG TCC CA-3′ (22). Thermal cycling was performed with the following programs: either 30 or 35 cycles of heat denaturation at 94°C for 1 min, annealing at 55°C for Vα13/JαDO, 52°C for Vα14/Jα281, 54°C for Cα-rev1 for 2 min, and elongation at 72°C for 1 min. PCR products were electrophoresed on a 3.0% agarose ethidium bromide gel.
RESULTS
The Expression of NK1.1 on Thymocytes and Spleen Cells from (B6 × DO10)F1 and (D2 × DO10)F1 Mice.
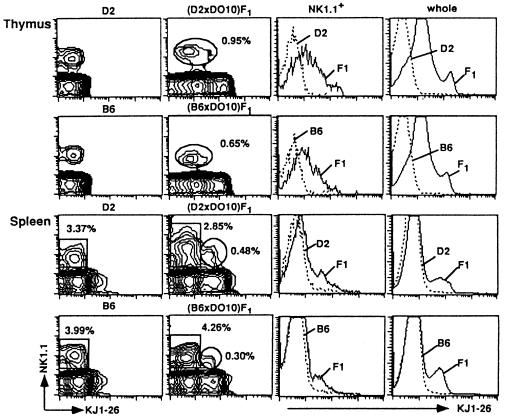
Cells from DO10 mice that have been established by serial backcross to BALB/c mice express no NK1.1 molecules. Thus, we first confirmed whether the NK1.1 Ag is expressed on thymocytes and spleen cells from (B6 × DO10)F1 and (D2 × DO10)F1 mice. Fig. 1 illustrates the representative profile of FACS analysis of thymocytes and spleen cells from two (D2 × DO10)F1 and four (B6 × DO10)F1 mice (16 wk old) examined separately. The profiles of the negative control mice (D2 and B6) for KJ1-26 (anti-DO11.10 α/β TCR clonotype monoclonal antibody) expression are also shown. No NK1.1+ cells were detected in the thymocyte and splenocyte populations of DO10 mice (data not shown). By contrast, normal proportions of NK1.1+KJ1–26+ thymocytes were observed in the thymuses of (D2 × DO10)F1 and (B6 × DO10)F1 mice (Fig. 1) that were comparable with those of NK1.1+α/β TCR+ thymocytes in normal mice such as B6 mice of the same age (1–3, 10). Substantial proportions of NK1.1+ KJ1-26+ cells were also detected in spleens of (D2 × DO10)F1 and (B6 × DO10)F1 mice (Fig. 1). It is also shown in this figure most NK1.1+ thymocytes are dull to intermediate positive for KJ1-26 staining.
Figure 1.
Analysis of NK1.1 expression on thymocytes and spleen cells from D2, B6, (D2 × DO10)F1, and (B6 × DO10)F1 mice. Thymocytes and splenocytes were stained with PE-anti-NK1.1 and biotin-KJ1-26 followed by Streptavidin-FITC. The proportions of NK1.1+KJ1-26+ cells (thymus) and NK1.1+KJ1-26− and NK1.1+ KJ1-26+ cells (spleen) of (D2 × DO10)F1 and (B6 × DO10)F1 mice are indicated (Middle Left). Fluorescence intensities of KJ1-26 among the NK1.1+ population in the thymus as well as in whole thymocyte and splenocyte populations are also shown in the histograms to compare those between D2 and (D2 × DO10)F1 or between B6 and (B6 × DO10)F1, respectively (Right).
Phenotype and TCR Expression of NK1.1+ Thymocytes from (B6 × DO10)F1 and (D2 × DO10)F1 Mice.
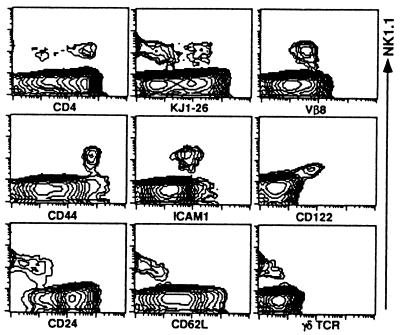
We then analyzed various surface markers on NK1.1+ thymocytes from (B6 × DO10)F1 and (D2 × DO10)F1 mice. Fig. 2 shows a representative FACS profile of thymocytes from three (B6 × DO10)F1 mice examined separately. The NK1.1+ thymocytes consist of CD4− and CD4+ subpopulations. Most of the NK1.1+ thymocyte population expresses intermediate levels of Vβ8 and low to intermediate levels of KJ1-26. It should be noted in this figure that KJ1-26low and KJ1-26intermediate cells seemed to form two distinct subpopulations. These findings suggest that expression of Vα14 is not requisite for NK1.1+ α/β TCR+ cells to be positively selected at least in the thymus. Furthermore, the NK1.1+ thymocytes showed characteristics (CD44high, ICAM-1+, CD122 (IL-2Rβ)high, CD24 [heat stable Ag]low, and CD62Llow) similar to those of normal B6 or B10 background mice (3). No γ/δTCR+ cells were detected among the NK1.1+ thymocyte populations. Almost identical results were obtained with NK1.1+ thymocytes from (D2 × DO10)F1 mice (data not shown).
Figure 2.
Phenotypic analysis of thymocytes from (B6 × DO10)F1 mice. Thymocytes were stained either with combination of PE-anti-NK1.1 and biotin-Abs (KJ1-26, anti-Vβ8, CD44, ICAM-1, CD24, CD62L, and γ/δTCR) plus Streptavidin-FITC or PE-anti-CD122 and biotin-anti-NK1.1 plus Streptavidin-FITC. Expression of NK1.1 (Vertical) is indicated. Other markers on thymocytes (Horizontal) are indicated.
To examine the time course of appearance of the NK1.1+ thymocytes in an ontogenic perspective, we analyzed thymocytes from (B6 × DO10)F1 mice at various ages (11, 12, 16, 24, and 32 wk). Approximately 0.9, 2.8, and 4.2% of the thymocytes were NK1.1+ at 12, 16, and 32 wk of age, respectively (data not shown). These findings indicate that NK1.1+ thymocytes from TCR Tgm gradually increased with age as we had reported earlier with normal mice (3). Thus, the NK1.1+ α/βTCR+ thymocytes, which exhibit almost identical characteristics to those observed in normal mice, have been generated normally in mice bearing Vα13/Vβ8.2.
IL-4 Production by Spleen Cells from (B6 × DO10)F1 and (D2 × DO10)F1 Mice After Administration of Anti-CD3 or KJ1-26.
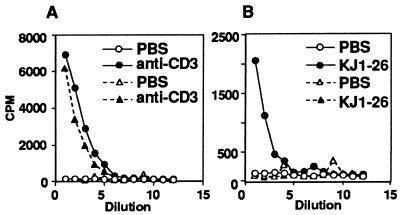
We then analyzed functionally the presence of NK1.1+ α/βTCR+ cells in the spleen of (B6 × DO10)F1 and (D2 × DO10)F1 mice using the IL-4 production assay of Yoshimoto and Paul (20). After short-term stimulation with anti-CD3 monoclonal antibody in vivo, spleen cells from these F1 mice produced significant amounts of IL-4 (Fig. 3A). These findings indicate that functional NK1.1+α/βTCR+ cells are present in the spleens of (B6 × DO10)F1 and (D2 × DO10)F1 mice. By contrast, stimulation with KJ1-26 in vivo resulted in substantial IL-4 production in spleen cells of (D2 × DO10)F1 mice but not in those of (B6 × DO10)F1 mice (Fig. 3B). This finding suggests that splenic NK1.1+α/βTCR+ cells have been anergized against stimulation through TCR in the mice with a negative-selecting background (23), (B6 × DO10)F1 mice.
Figure 3.
Induction of IL-4 by in vivo administration of anti-CD3 or KJ1-26. (D2 × DO10)F1 and (B6 × DO10)F1 mice were injected intravenously with 4 μg of anti-CD3 or KJ1-26, and spleens were removed after 90 min. Spleen cells (5 × 106) were cultured in 96-well plates for 2 h. Supernatants were harvested, and IL-4 was measured using the CT.4S cells. (A) Induction of IL-4 in response to in vivo treatment with anti-CD3. (B) Induction of IL-4 in response to in vivo treatment with KJ1-26. (D2 × DO10)F1 mice were injected with PBS (○) or anti-CD3 or KJ1-26 (•). (B6 × DO10)F1 mice were injected with PBS (▵) or anit-CD3 or KJ1-26 (▴).
NK1.1+ Thymocytes in Rag-1−/−/DO10 Mice.
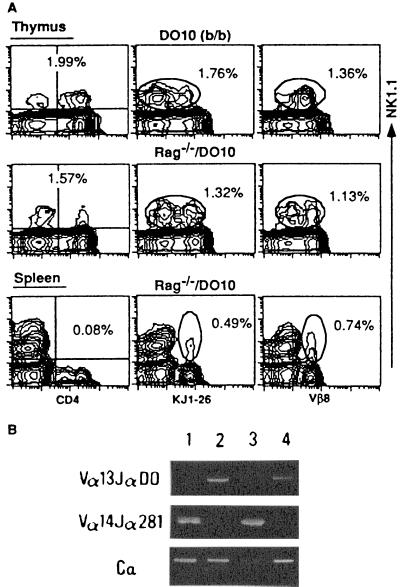
It has been reported that allelic exclusion of TCRα locus sometimes is incomplete (13). To exclude the possibility that in (B6 × DO10)F1 and (D2 × DO10)F1 mice intrinsic Vα14 is expressed on the NK1.1+α/βTCR+ cells in association with the transgenic Vβ8.2, we then analyzed thymocytes and spleen cells from Rag-1−/−/DO10 mice. Fig. 4A shows that almost identical populations of NK1.1+Vα13/Vβ8.2+ cells as seen in (B6 × DO10)F1 and (D2 × DO10)F1 mice (Figs. 1 and 2) are present in the thymuses of both Rag-1−/−/DO10 and control DO10 (b/b) mice, which were prepared by backcross of (B6 × DO10)F1 mice with B6 mice several times so they possessed the H-2b/b type. It should be noted in this figure that both NK1.1+ KJ1-26low and NK1.1+ KJ1-26intermediate cells are detected in the thymus of Rag-1−/− DO10 mice. A significant proportion of NK1.1+KJ1-26+ or NK1.1+Vβ8+ cells was also detected in the spleen of Rag-1−/−/DO10 mice as was shown in (B6 × DO10)F1 and (D2 × DO10)F1 mice (Fig. 1). It seemed that these NK1.1+ splenic cells reside in a CD4− population (Fig. 4A). It is also shown in Fig. 4B that Vα14-Jα281 transcripts are not detected in thymocytes of Rag-1−/−/DO10 mouse with 35 cycles of PCR amplification. Thus, the KJ1-26low population in NK1.1+ thymocytes seems to express no Vα14-Jα281. It seems that the low expression of KJ1-26 on Rag-1−/−/DO10 thymocytes is not due to Vα14-Jα281 pairing to the Vβ chain of DO10 TCR. These findings permit us to conclude that the NK1.1+α/βTCR+ cells have indeed developed in the thymus and spleen in the absence of Vα14-Jα281.
Figure 4.
NK1.1 expression and Vα usage on thymocytes and splenocytes from DO10 (H-2b/b) and Rag-1−/−/DO10 mice. (A) FACS analysis of NK1.1 expression on thymocytes and splenocytes. Cells were stained with PE-anti-NK1.1 and FITC-anti-CD4, biotin-KJ1-26, or biotin-anti-Vβ8 followed by Streptavidin-FITC. The proportions of whole NK1.1+ cells are shown in the thymus, and NK1.1+CD4+, NK1.1+KJ1-26+, and NK1.1+Vβ8+ cells are shown in the spleen. (B) Analysis of TCRα chain usage in thymocytes and splenocytes using reverse transcription-PCR. Vα13-Jα DO (DO10 TCRα), Vα14-Jα281, and Cα transcripts were amplified with specific primers as described in Materials and Methods from B6 thymocytes (lane 1), Rag-1−/−/DO10 thymocytes (lane 2), B6 splenocytes (lane 3), and Rag-1−/−/DO10 splenocytes (lane 4), respectively.
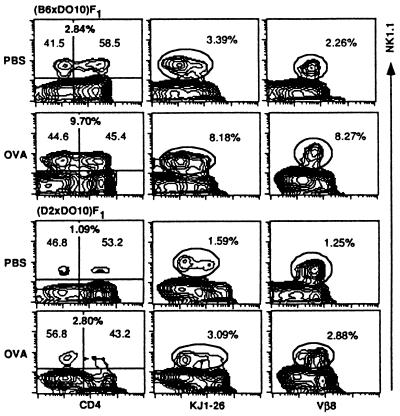
Influence of Administration of cOVA on NK1.1+ α/βTCR+ Thymocytes in (B6 × DO10)F1 and (D2 × DO10)F1 Mice.
A previous study (15) showed that KJ1-26+ CD4+8+ double-positive immature thymocytes of DO10 mice were deleted after administration of cOVA in vivo. We examined whether the Ag-specific clonal deletion occurred similarly among the NK1.1+ thymocyte population from (B6 × DO10)F1 (negative selecting background) or (D2 × DO10)F1 (positive selecting background) mice (16 wk old) after administration of the specific Ag, cOVA (23). The total number of thymocytes decreased markedly in (B6 × DO10)F1 (86.7%) and (D2 × DO10)F1 mice (95.3%) after injection with cOVA as compared with those of control mice injected with PBS alone. The reduction was most prominent in the double positive population, especially in (D2 × DO10)F1 mice (99.2%). These findings are quite compatible with those reported earlier in thymocytes of DO10 mice (15). We then analyzed the proportions of NK1.1+ population in the whole thymocyte population and compared these values between cOVA-injected mice and control F1 mice. Fig. 5 shows that the proportion of NK1.1+ thymocytes was markedly increased in the cOVA-injected mice as compared with that of control mice. The proportion of NK1.1+ thymocytes was generally high in (B6 × DO10)F1 mice given either PBS or cOVA as compared with those in (D2 × DO10)F1 mice. It seemed that the NK1.1+ thymocytes were less influenced by cOVA injection than the major population of NK1.1− thymocytes. When expression of α/βTCR [Vβ8 and clonotypic TCR (KJ1-26)] on the NK1.1+ thymocytes was analyzed, actually all remaining NK1.1+ thymocytes from both cOVA- injected (B6 × DO10)F1 or cOVA-injected (D2 × DO10)F1 mice and those of control F1 mice were Vβ8+ and KJ1-26low or KJ1-26intermediate. Thus, it seemed that the administration of cOVA resulted in no alteration of the expression pattern of Vα13/Vβ8.2.
Figure 5.
Influence of cOVA administration on NK1.1+ thymocytes in (B6 × DO10)F1 and (D2 × DO10)F1 mice. Thymocytes from cOVA- or PBS-treated mice were isolated and stained with PE-anti-NK1.1, FITC-anti-CD4, and biotin-KJ1-26 or biotin-anti-Vβ8 followed by Streptavidin-FITC. A representative profile from three separate experiments is shown. The proportions of NK1.1+, NK1.1+ CD4−, and NK1.1+ CD4+ cells (Left), NK1.1+KJ1-26+ (NK1.1+KJ1-26low and NK1.1+KJ1-26intermediate) (Middle), and NK1.1+Vβ8+ (Right) cells are indicated.
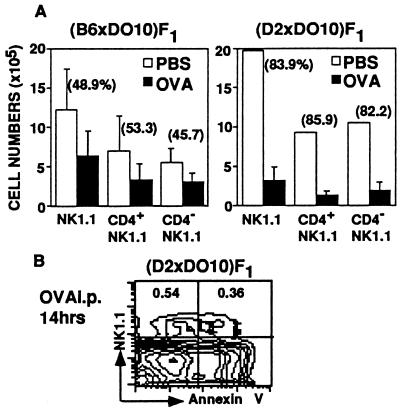
Then, the total numbers of NK1.1+ thymocytes from cOVA-injected (B6 × DO10)F1 and (D2 × DO10)F1 mice and PBS-injected F1 mice were calculated and compared between the cOVA-injected and control F1 mice. Fig. 6A shows average numbers ± SD of three to five mice per each group and the percentage reduction of the cell number in experimental groups compared with that in control groups. The number of NK1.1+ thymocytes was significantly reduced in the cOVA-injected mice as compared with that of control mice. These findings demonstrate that administration of cOVA induces a significant deletion of both NK1.1+ KJ1-26low and KJ1–26intermediate thymocytes in the (B6 × DO10)F1 and (D2 × DO10)F1 mice. When thymocytes from (D2 × DO10)F1 mice were stained with Annexin-V-FITC and PK136 14 h after cOVA injection, almost half of NK1.1+ thymocytes were found to be Annexin-V positive and thus undergoing apoptosis (Fig. 6B).
Figure 6.
Clonal deletion of NK1.1+ thymocytes following cOVA administration. (A) The total number of NK1.1+ thymocytes from cOVA- or PBS-injected (B6 × DO10)F1 and (D2 × DO10)F1 mice. Absolute cell numbers of NK1.1+, NK1.1+CD4+, and NK1.1+CD4− thymocytes in mice given cOVA (closed bar) or PBS (open bar) are shown. Results represent means and SD of the calculated cell numbers (n = 3–5). Percent reduction is also indicated. (B) Expression of an early apoptotic marker, Annexin V, on NK1.1+ thymocytes obtained from (D2 × DO10)F1 mice given cOVA. Thymocytes were stained with Annexin V-FITC and NK1.1-PE, and the proportions of NK1.1+ Annexin V− and NK1.1+ Annexin V+ cells are indicated.
The reduction, however, was more prominent in (D2 × DO10)F1 thymocytes than in (B6 × DO10)F1 thymocytes. Perhaps the negative selecting background [including anergy induction (Fig. 3)] and the resultant low expression of KJ1-26 on the NK1.1+ cells of (B6 × DO10)F1 mice were the basis of the low efficiency of clonal elimination of the NK1.1+ thymocytes.
DISCUSSION
It has been demonstrated that murine NK1.1+α/βTCR+ (NK-T) cells express an invariant TCR α chain (Vα14-Jα281) in association with biased Vβ chains, predominantly Vβ8.2 (1–5, 11, 12, 24). The NK1.1+α/βTCR+ cells seem to be selected positively by class Ib, CD1 molecules (1, 4, 6–9, 22, 25, 26). In the present study, we analyzed mainly the development and negative selection of the NK1.1+α/βTCR+ thymocytes using Tgm bearing Vα13/Vβ8.2 that is specific for cOVA and restricted to I-Ad (15, 17, 18). We found that a normal population of NK1.1+α/βTCR+ thymocytes was generated in the thymuses of (B6 × DO10)F1 and (D2 × DO10)F1 mice. A possibility of expression of intrinsic Vα14-Jα281 was excluded by an experiment in which Rag-1−/−/DO10 thymocytes were analyzed.
However, when the actual number of NK1.1+α/βTCR+ cells was compared between (B6 × DO10)F1 and (D2 × DO10)F1 mice, the number was markedly larger in the latter (D2 × DO10)F1 mice (positive selecting background) than that in the (B6 × DO10)F1 mice (negative selecting background) (23). We found that spleen cells from (B6 × DO10)F1 mice produced IL-4 after short-term exposure to anti-CD3 monoclonal antibody in vivo but not after exposure to KJ1-26 monoclonal antibody. On the other hand, spleen cells from (D2 × DO10)F1 mice produced IL-4 after stimulation with either anti-CD3 or KJ1-26 monoclonal antibody in vivo. Treatment of the spleen cells with PK136 plus complement before IL-4 assay abolished completely the production of IL-4 (data not shown). Thus, NK1.1+α/βTCR+ cells seemed to differentiate normally in (D2 × DO10)F1 mice but to be functionally influenced by the H-2b products in (B6 × DO10)F1 mice. Perhaps large proportions of both NK1.1+ and NK1.1− cells have been either deleted or anergized by H-2b products during differentiation in the (B6 × DO10)F1 mice. These findings reveal that both NK1.1− and NK1.1+ populations of Vα13/Vβ8.2+ T cells have indeed undergone negative selection under the influence of H-2b products.
Most of the NK1.1+α/βTCR+ thymocytes from (B6 × DO10)F1, (D2 × DO10)F1, and Rag-1−/−/DO10 mice expressed low or intermediate levels of clonotypic TCR (Vα13/Vβ8.2), although the expression of Vβ8.2 seemed to be uniform (intermediate). Abo et al. (5) reported that the two-peak pattern of α/βTCR was characteristic of NK1.1+α/βTCR+ cells in the thymus and liver but not those in other lymphoid tissues. Furthermore, these NK1.1+ Vα13/Vβ8.2+ thymocytes showed ontogenic and phenotypic characteristics identical to those detected in normal (wild-type) mice. Thus, the NK1.1+α/βTCR+ thymocytes seemed to differentiate normally, and the normal proportion of these thymocytes was generated in the absence of Vα14-Jα281 expression.
Recently Schulz et al. (13) reported that in anti-H-Y/Rag-2−/− Tgm, normal generation of NK1.1+ Vα3/Vβ8.2+ thymocytes was observed. It seems that in our TCR Tgm system and that of Schulz et al. (13), a prerequisite role of Vα14-Jα281 expression may not be essential. This finding seems to stand in marked contrast to those reported by Taniguchi et al. (11) and Bendelac et al. (12). Using Vα14-Jα281 Tgm, these authors demonstrated that expression of the Vα14-Jα281 biased the differentiation of NK1.1− major thymocytes toward the NK-T developmental pathway.
The difference in the role for Vα14-Jα281 on the generation of NK-T cells seen in previous studies (11, 12) and those of Schulz et al. (13) or ours cannot be explained at present. We reason that irrespective of Vα chains, the expression of Vβ8.2 may be sufficient for the NK1.1+α/βTCR+ thymocytes to develop toward a normal population size. Bendelac et al. (7) reported that NK1.1+ Vα3.2+ Vβ8.2+ cells were present in the CD4+ population. In addition, an essential requirement for expression of certain Vβs (Vβ8.2, Vβ7, and Vβ2) on development of the liver NK-T cells was shown by Ohteki and MacDonald (27). It seems to us that the functional role of Vα14 expression in generation of NK1.1+α/βTCR+ cells is different between the thymocyte population and extrathymic cell populations (28).
In the present study, we found that approximately one-half to approximately 85% of the NK1.1+α/βTCR+ thymocytes of (B6 × DO10)F1 or (D2 × DO10)F1 mice, respectively, were deleted after exposure in vivo to the specific Ag, cOVA. Thus, it was shown again that the NK1.1+α/βTCR+ cells undergo negative selection in the presence of specific Ag, even though, especially in (B6 × DO10)F1 mice, the extent of clonal elimination was not as complete as that observed in NK1.1− major thymocytes. Perhaps this difference between (B6 × DO10)F1 and (D2 × DO10)F1 mice resulted from the amounts of I-Ad expressed in these mice [(H-2b × H-2d) versus (H-2d × H-2d)] as well as the presence of an H-2b influence [negative selecting background (15, 21)] of (B6 × DO10)F1 mice as described above.
At any rate, the present findings seem to be somewhat inconsistent with a previous report by Schulz et al. (13). These authors showed that negative selection NK-T cells did not function in male Tgm bearing TCR specific for a male-specific peptide plus H-2Db. The difference in TCR [class II restricted in the present study versus class I-restricted in (13)], Ag (soluble cOVA versus H-Y antigen expressed on the cell) and affinity between the TCR and Ag may be the basis of these contradictory observations between our present study and that by Schulz et al. (13). Indeed, Curnow et al. (29) showed that α/βTCR+ CD4, 8 double negative cells with NK1.1 expression from TCR-Tgm that react with allo-class I major histocompatibility complex (Kb) independent of CD8 (high-affinity TCR) were deleted in the H-2k/b background, but those from the other TCR-Tgm that react with the same Kb only in the presence of CD8 (low-affinity TCR) were not deleted. These findings seem to be compatible with the prospect that the affinity/avidity of TCR–major histocompatibility complex–peptide interaction may determine the selection pattern (positive, negative, or neutral) of NK1.1+α/βTCR+ thymocytes as well as that of the major NK1.1− thymocyte population.
In this connection, we demonstrated previously that negative selection of Vβ6+ cells that are reactive to I-E plus minor lymphocyte stimulatory-1a (Mls-1a) Ag occurred among the CD44+ CD4+8− heat-stable Ag− thymocyte population (almost the same population as NK1.1+ T cells among CD4+8− thymocyte subpopulation) in I-E+ Mls-1a mice, whereas the elimination of Vβ8.1+ cells that are also reactive to I-E plus Mls-1a was not efficiently induced (3). On the other hand, the complete elimination of both Vβ6+ and Vβ8.1+ cells was demonstrated among the CD44− CD4+8− heat-stable Ag− major thymocyte population. Similar findings among double negative α/βTCR+ cells were reported by Huang and Crispe (30) and Takahama et al. (31) in the Mls-1a or staphylococcal endotoxin B-system. Thus, it seems that intrathymic negative selection does not eliminate all NK1.1+α/βTCR+ thymocytes reactive to self-Ag. In our subsequent studies (32, 33), we demonstrated that under certain conditions deletion or activation of Vβ6+ T cells was induced by I-E plus Mls-1a, but those of Vβ8.1+ T cells did not occur efficiently. The avidity of the particular TCR to the tolerogens may determine whether complete elimination of the Ag-reactive TCR repertoire is accomplished or insufficient elimination is induced in the NK1.1+α/βTCR+ thymocytes (34). The TCR derived from DO10 may possess sufficient affinity to cOVA plus I-Ad for NK1.1− major thymocytes but not for all NK1.1+ thymocytes to be eliminated. On the basis of the findings of Huang and Crispe (30), those of Takahama et al. (31), and ours (3), we considered that the NK1.1+α/βTCR+ thymocytes indeed undergo negative selection, but the efficiency may be low as compared with that of the NK1.1− major thymocyte population.
Concerning the different efficacy of negative selection seen between NK1.1+ and NK1.1− thymocytes, the density of the TCR on the NK1.1+ thymocytes may not reach the levels that transduce sufficient signaling to lead to complete elimination of the NK1.1+α/βTCR+ thymocytes (34). In addition, the difference in efficiency of clonal elimination between (B6 × DO10)F1 and (D2 × DO10)F1 mice seen in the present study, both of which were given cOVA, seemed to reflect differences in the amounts of TCR expressed on NK1.1+α/βTCR+ thymocytes.
Acknowledgments
We thank Dr. D. Y. Loh for providing us with DO11.10 TCR mouse and Dr. P. Lucas for advice on screening transgenic mice with PCR. We also thank Ms. Michiyo Konishi, Ms. Maki Sato, Ms. Atsuko Takano, and Ms. Tazim Verjee for their secretarial assistance with the manuscript. This study was supported in part by a grant-in-aid for scientific research, The Special Grant-in-Aid-for Promotion of Education and Science in Hokkaido University provided by The Ministry of Education, Science, Sports and Culture, Japan, and grants from the Hokkaido Foundation for the Promotion of Scientific and Industrial Technology, the Tomakomai East Hospital Foundation, the Nishimura Aging Fund, and U. S. Public Health Service-National Institutes of Health Institute on Aging Grant AG05628-13 to R.A.G.
ABBREVIATIONS
- Ag
antigen
- cOVA
chicken ovalbumin
- DO10 mouse
DO11.10 TCR mouse
- FITC
fluorescein isothiocyanate
- IL
interleukin
- Mls-1a
minor lymphocyte stimulatory-1a
- PE
phycoerythrin
- TCR
T cell receptor
- Tgm
transgenic mouse
References
- 1.Bendelac A. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald H R. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arase H, Arase N, Ogasawara K, Good R A, Onoé K. Proc Natl Acad Sci USA. 1992;89:6506–6510. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Proc Natl Acad Sci USA. 1995;92:1200–1204. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abo T, Ohteki T, Seki S, Koyamada N, Yoshikai Y, Masuda T, Rikiishi H, Kumagai K. J Exp Med. 1991;174:417–424. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coles M C, Raulet D H. J Exp Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac A, Lantz O, Quimby M E, Yewdell J W, Bennink J R, Brutkiewicz R R. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 8.Bendelac A. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emoto M, Emoto Y, Kaufmann H E. Int Immunol. 1995;7:1729–1739. doi: 10.1093/intimm/7.11.1729. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa K, Iwabuchi K, Ogasawara K, Ato M, Kajiwara M, Nishihori H, Iwabuchi C, Ishikura H, Good R A, Onoé K. Proc Natl Acad Sci USA. 1997;94:2472–2477. doi: 10.1073/pnas.94.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendelac A, Hunziker R D, Lantz O. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz R-J, Parkes A, Mizoguchi E, Bhan A, Koyasu S. J Immunol. 1996;157:4379–4389. [PubMed] [Google Scholar]
- 14.Von Boehmer H, Kirberg J, Rocha B. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 16.Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoé K. J Exp Med. 1994;180:423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White J, Haskins K, Marrack P, Kappler J. J Immunol. 1983;130:1033–1037. [PubMed] [Google Scholar]
- 18.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unkeless J C. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y-H, Chiu N M, Mandel M, Wang N, Wang C-R. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu C-P, Kappler J W, Marrack P. J Exp Med. 1996;184:1619–1630. doi: 10.1084/jem.184.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arase H, Arase-Fukushi N, Good R A, Onoé K. J Immunol. 1993;151:546–555. [PubMed] [Google Scholar]
- 25.Smiley S T, Kaplan M H, Grusby M J. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 26.Mendiratta S K, Martin W D, Hong S, Boesteanu A, Joyce S, Kaer L V. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 27.Ohteki T, MacDonald H R. J Exp Med. 1996;183:1277–1282. doi: 10.1084/jem.183.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lantz O, Bendelac A. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curnow S J, Boyer C, Buferne M, Schmitt-Verhulst A-M. Immunity. 1995;3:427–438. doi: 10.1016/1074-7613(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Crispe I N. J Exp Med. 1992;176:699–706. doi: 10.1084/jem.176.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahama Y, Kosugi A, Singer A. J Immunol. 1991;146:1134–1141. [PubMed] [Google Scholar]
- 32.Arase N, Arase H, Takayanagi T, Mishima M, Iwabuchi K, Ogasawara K, Onoé K. Immunobiology. 1995;193:378–390. doi: 10.1016/S0171-2985(11)80425-0. [DOI] [PubMed] [Google Scholar]
- 33.Arase-Fukushi N, Arase H, Ogasawara K, Good R A, Onoé K. J Immunol. 1993;151:4445–4454. [PubMed] [Google Scholar]
- 34.Budd R C, Mixter P F. Immunol Today. 1995;16:428–431. doi: 10.1016/0167-5699(95)80019-0. [DOI] [PubMed] [Google Scholar]