Abstract
The role of processing in antigen (Ag) presentation and T cell activation in experimental allergic encephalomyelitis (EAE) was evaluated in wild-type mice, mice that selectively express either Ii p31 or p41, and mice completely deficient in Ii or H-2M. We demonstrate that processing of myelin oligodendrocyte glycoprotein (MOG) is required for presentation of the dominant encephalitogenic MOG epitope, p35-55. Ii p31- and p41-expressing mice developed EAE with similar incidence to wild-type mice, although p41 mice had a more severe course. Ag-presenting cells (APCs) from Ii- or H-2M–deficient mice could present p35-55, but not MOG, demonstrating that these APCs could not process native MOG. Ii- and H-2M–deficient mice were not susceptible to EAE by immunization with p35-55 or MOG or by adoptive transfer of encephalitogenic T cells. However, CD4+ T cells from p35-55–immunized H-2M–deficient mice proliferated, secreted IFN-γ, and transferred EAE to wild-type, but not H-2M–deficient, mice. Thus, EAE resistance in H-2M–deficient mice is not due to an inability of APCs to present p35-55, or an intrinsic defect in the encephalitogenic T cell repertoire, but reflects a defect in APC function. Our results indicate that processing is required for initial Ag presentation and CNS T cell activation and suggest that autopathogenic peptides of CNS autoantigen may not be readily available for presentation without processing.
Introduction
Experimental allergic encephalomyelitis (EAE) is an inflammatory demyelinating CNS disease that serves as a model for multiple sclerosis (MS) and other organ-specific T cell–mediated autoimmune diseases (1, 2). Activated CD4+ Th1 cells that recognize CNS self-antigens (autoantigens) mediate EAE and are thought to have a central role in MS pathogenesis (2). Activation of CD4+ T cells, which occurs when a specific Ag is presented in association with MHC class II molecules expressed on the surface of antigen presenting cells (APCs), may be required at different stages in the pathogenesis of CNS inflammatory disease (3–5). Ag presentation by APCs outside the CNS can lead to T cell activation, a prerequisite for T cell entry into the CNS (3, 4). Ag presentation by resident CNS accessory cells, which are nonprofessional APCs, may be necessary for recognition of CNS autoantigen and T cell activation during the initial CNS inflammation (3) and T cell activation during progression from acute to chronic and relapsing stages of CNS demyelinating disease (5). In general, Ag presentation by APCs requires “processing,” which involves proteolytic Ag degradation in endocytic/lysosomal compartments and association of peptide cleavage products with class II molecules before display on the surface of APCs (6). In vitro studies have established that endocytic processing of the native form of the CNS autoantigen, myelin basic protein (MBP), is necessary for presentation to encephalitogenic CD4+ T cells (7). If peptides of myelin autoantigens are not readily accessible for Ag presentation in normal CNS, endocytic processing by CNS APCs may be required for initial Ag presentation and T cell activation in vivo.
Invariant chain (Ii) and H-2M (HLA-DM) are molecules involved in class II biosynthesis and endocytic processing (8). Ii serves as a chaperone, directing transport of newly formed class II α and β chains to the endosomal/lysosomal compartment (8). Two isoforms of Ii are expressed, p31 and p41 (9). Although Ii p31 and p41 both facilitate class II assembly and prevent premature Ag binding to class II molecules (8), some in vitro data have indicated that Ii p41 may modulate the proteolytic environment of the endocytic compartment and confer selective advantage in Ag presentation to CD4+ T cells (10–12). As Ii p31 and p41 are proteolytically cleaved, the residual peptide fragment, CLIP (class II–associated invariant chain peptide), remains associated to class II molecules (13). H-2M, a nonclassical class II molecule, facilitates the removal of CLIP, permitting the binding of peptide Ag’s for T cell presentation (14). Deficiencies in either Ii or H-2M, can cause defects in Ag processing (8), and have been instrumental in evaluating the requirements for intracellular peptide loading and Ag presentation in vivo (15–20).
In this investigation we evaluated the role of Ag processing in EAE by examining the T cell response to myelin oligodendrocyte glycoprotein (MOG) in mice that selectively express Ii p31 or p41 and mice that are completely deficient in Ii or H-2M molecules. We established that processing of native MOG by APC is required for presentation of the dominant encephalitogenic MOG peptide, p35-55. Although both Ii p31 and p41 mice were susceptible to EAE, Ii p41 mice sustained a more severe course. Our vivo studies utilizing mice completely deficient in Ii or H-2M indicate that processing is required for Ag presentation during the induction of T cell–mediated CNS inflammation. Results in this study indicate that peptides of this CNS autoantigen may not be available for class II restricted Ag presentation in normal CNS without processing. Thus, reagents that selectively interfere with endocytic processing could be beneficial for immunotherapy in Ag-specific CNS autoimmune disease.
Methods
Mice.
Characterization of mice deficient in Ii, Ii p31, Ii p41, and H-2M has been described (15, 18, 21, 22). Deficiency in each of these genes has been bred onto the C57BL/6 background for four generations. Wild-type C57BL/6 mice, Ii-deficient mice, and H-2M–deficient mice used in adoptive transfer studies were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA).
Antigens, antibodies, and recombinant cytokines.
Mouse MOG peptide 35-55 (MEVGWYRSPFSRVVHLYRNGK) (23) was synthesized and HPLC purified by QCB Inc. (Hopkinton, Massachusetts, USA). Whole MOG was purified from mouse brain as described previously (23) and full-length rMOG (amino acid residues 1–218) was a kind gift of C. Bernard (Latrobe University, Melbourne, Australia).
Induction and clinical evaluation of EAE.
Mice received a subcutaneous injection in the flank of 100 μg of mouse MOG p35-55 in 0.1 ml of PBS emulsified in an equal volume of CFA supplemented with 2 mg/ml of mycobacterium tuberculosis H37RA (MT; DIFCO Laboratories, Detroit, Michigan, USA). Immediately thereafter and again 48 hours later, mice received an intravenous injection of 150 ng of pertussis toxin (PT) in 0.2 ml of PBS. For adoptive transfer of EAE, p35-55–specific T cells were activated with APCs and p35-55 3 days before transfer. After Ficoll, cells were washed three times and counted. Recipient mice received 5 × 106 cells in 0.5 ml PBS intravenously. Mice were monitored for symptoms of EAE daily and scored as follows: 0, no disease; 1, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb plus forelimb paralysis; 5, moribund.
Histological evaluation of tissue.
At the completion of the experiment on day 30, mice were anesthetized by isoflurane inhalation and perfused with PBS containing 4% (vol/vol) paraformaldehyde. Fixed CNS tissues were embedded in paraffin wax, cut in 5-μm sections, and stained with hematoxylin and eosin. Stained sections were examined in a blind fashion as described previously (23).
Proliferation assays.
Cells were cultured in serum-free medium, X-Vivo 20 (BioWhittaker Inc., Walkersville, Maryland, USA) supplemented with 5 × 10–5 M 2-mercaptoethanol, 2 mM glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin. A total of 1 × 104 MOG p35-55–specific CD4+ T cells were cultured with 5 × 105 γ-irradiated splenic APC and MOG peptide p35-55 or intact MOG in 0.2 ml culture media in 96-well microtiter plates (Falcon Labware, Oxnard, California, USA). At 48 hours, each well was pulsed with 1 μCi [3H]thymidine and harvested 16 hours later. In APC fixation experiments, 5 × 105 splenic APCs were treated with 0.05% paraformaldehyde for 30 minutes at room temperature, washed three times, and cultured with 1 × 104 MOG p35-55–specific T cells in the presence of either p35-55 or intact MOG. For primary proliferative responses, 5 × 105 spleen cells removed from immunized mice were cultured in 0.2 ml media with p35-55 or native MOG. Cultures were pulsed at 72 hours with 1 μCi [3H]thymidine and harvested 16 hours later. In experiments using chloroquine, APCs were incubated with 75 μM chloroquine for 75 minutes and removed. After incubation with Ag for 30 minutes, MOG-specific T cells were added, pulsed at 72 hours, and harvested 16 hours later.
Cytokine analysis.
Cell culture supernatants were collected at 24-hour incubation for cytokine analysis. Quantitative ELISA for IL-2, IL-4, and IFN-γ were performed using paired mAb’s specific for corresponding cytokines per manufacturer’s recommendations (PharMingen, San Diego, California, USA).
RT-PCR.
Mice were perfused with PBS during euthanasia to remove circulating blood cells. Total RNA was then extracted with TRIZOL (GIBCOBRL; Life Technologies Inc., Rockville, Maryland, USA). RT and PCR were performed using the Access RT-PCR system from Promega Corp. (Madison, Wisconsin, USA) using one cycle: 48°C for 45 minutes followed by 94°C for 2 minutes. The following oligonucleotide primers (designed from published murine cDNA sequences; refs. 9, 24) were purchased from Operon (Alameda, California, USA): CIITA, 5′-(CCCTGCGTGTGATGGATGTC) and 5′-(GTTGCCCTTAGCGTCTTCAG); Ii, 5′-GAGGCTAGAGCCATGGATGAC-3′ and 5′(3′ AGATGCTTCAGATTCTCTGGG-3′); H-2Ma, 5′-CTACGAGATGTTGATGCGGGAAGT-3′and 5′GTGTAGCGGTCAATCTCGTGTGTC-3′; class II β -chain, 5′-GCTA-CTTCACCAACGGGACG-3′ and 5′-GCTCTTCAGGCTGGG-ATGCT-3′; β-actin, 5′-TGTGATGGTGGGAATG-GGTCAG-3′ and 5′-TTTGATGTCACGCACGATTTCC-3′. Primers for internal CIITA detect an expected 635-bp fragment; primers for Ii detect an expected 490-bp fragment; primers for an H-2Ma detect a 320-bp fragment; and primers for β-actin detect a 510-bp fragment. PCR was performed using 35 cycles of amplification: 94°C for 30 seconds, 62°C for 60 seconds, and 72°C for 120 seconds.
Results
Native MOG requires APC processing for presentation of the dominant encephalitogenic MOG peptide, p35-55.
Ii- and H-2M–deficient mice have separate defects in maturation of MHC class II molecules. APC from H-2M–deficient mice express normal levels of mature (compact) class II molecules that are loaded with CLIP and have defects in processing native Ag (17, 18). Although Ii-deficient APCs have defects in expression of class II molecules and processing native Ag’s, they are remarkably efficient at presenting certain peptide Ag’s (15, 16). To examine whether endocytic processing of native MOG is required for presentation of the dominant encephalitogenic determinant, p35-55 (23), we first tested whether splenic APCs from wild-type C57BL/6 mice, Ii-deficient mice, and H-2M–deficient mice were capable of presenting p35-55 and intact MOG to I-Ab–restricted p35-55–specific T cells. As shown in Figure 1a, Ii-deficient and wild-type APCs presented p35-55 equally well. H-2M–deficient APCs, which express class II molecules bound with CLIP, required more than one log higher p35-55 concentration to stimulate proliferation, which is consistent with other observations using H-2M–deficient APCs for presentation of exogenous peptide Ag’s (17, 18). In contrast, neither Ii-deficient nor H-2M–deficient APCs were very effective in presenting native MOG to p35-55–specific T cells compared with wild-type APC (Figure 1b). That Ii- and H-2M–deficient APCs could present p35-55, but not MOG, suggested that processing is required for presentation of native MOG. When tested at the same time, we observed that paraformaldehyde-fixed wild-type APC could present p35-55, but not native MOG (Figure 1c), which clearly established that processing is required for presentation of native MOG to p35-55–specific T cells. In addition, we observed that the lysosomal inhibitor, chloroquine, completely inhibited presentation of native MOG (Figure 1d), but only had a minor effect on presentation of p35-55, further confirming that the endocytic compartment participated in processing of MOG.
Figure 1.
Presentation of native MOG requires endocytic processing. Ii- or H-2M–deficient APCs can present p35-55 (a), but not native MOG (b), to encephalitogenic p35-55–specific T cells. (c) Paraformaldehyde-fixed wild-type APCs can present p35-55, but not native MOG, to p35-55–specific T cells. (d) Chloroquine-treated wild-type APCs can present p35-55 but do not present native MOG to p35-55–specific T cells. Irradiated splenocytes were cultured with p35-55–specific T cells in the presence of p35-55, native MOG, or no Ag, as described in Methods. Proliferative responses were measured by [3H]thymidine incorporation. For APC fixation, splenocytes were treated with paraformaldehyde, then cultured with p35-55–specific T cells in the presence of either p35-55, native MOG (50 μg/ml), or no Ag (see Methods). Irradiated wild-type splenocytes (b), tested at the same time as paraformaldehyde-fixed wild-type APCs (c), stimulated T cell proliferation using native MOG. For chloroquine treatment (d), APCs were treated with 75 μM chloroquine for 75 minutes before the addition of Ag and T cells (see Methods).
CNS expression of Ii and H-2M mRNA is upregulated during EAE.
When stimulated by IFN-γ, astroglia cells cultured in vitro upregulate Ii and H-2M and present CNS autoantigen to encephalitogenic T cells (7). These results suggested that expression of class II processing elements might be important in CNS Ag presentation. Thus, we examined Ii and H-2M expression in the CNS of wild-type mice with and without EAE. Ii expression was detected in the brain of mice with EAE, but was not detected in normal brain (Figure 2). Ii message was barely detectable in normal spinal cord, but was clearly upregulated in EAE. Constitutive H-2Ma expression was detected in the spinal cord and brain of control mice, although there was marked H-2Ma upregulation in these tissues during EAE. Constitutive levels of Ii and H-2M mRNAs were detected in the spleens of both normal and EAE mice.
Figure 2.
CNS expression of Ii and H-2M is upregulated during EAE. C57BL/6 mice were immunized with MOG peptide p35-55 on day 0 as described in Methods. Mice that developed EAE were perfused with PBS during euthanasia to remove circulating blood cells. Total RNA was extracted with trizol, and mRNA expression of each gene was detected by RT-PCR as described in Methods.
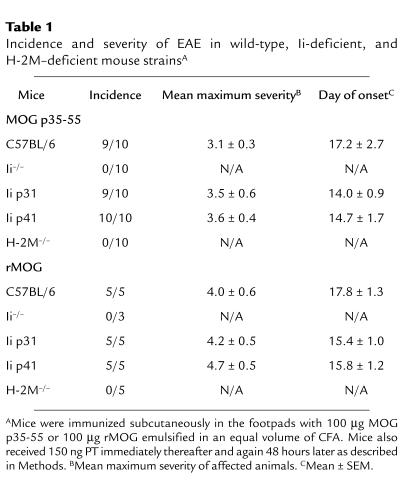
Ii p31 and p41 mice are susceptible to EAE, but mice completely deficient in Ii or H-2M are resistant.
Our observations that Ii- and H-2M–deficient APCs could present p35-55, but not native MOG, enabled us to use Ii- and H-2M–deficient mouse strains to evaluate the requirement for processing in Ag presentation in EAE. Ii-deficient and H-2M–deficient mice were first examined for EAE susceptibility by direct immunization with p35-55 or rMOG. In two separate experiments, none of the Ii-deficient or H-2M–deficient mice developed EAE (Table 1).
Table 1.
Incidence and severity of EAE in wild-type, Ii-deficient, and H-2M–deficient mouse strainsA
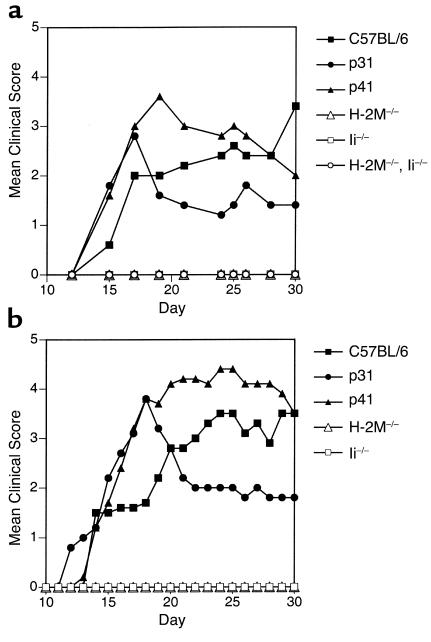
Some investigators have suggested that Ii p41 may be utilized more efficiently than Ii p31 in Ag processing and presentation (10–12). APCs in Ii p31 and p41 mice express normal levels of mature MHC class II molecules and these mice contain normal levels of CD4+ T cells (19). When tested for EAE susceptibility, we observed that Ii p31, Ii p41, and wild-type strains developed EAE with similar incidence when immunized with either p35-55 or rMOG (Table 1). However, after the initial onset of acute EAE, Ii p41 mice showed a more severe disease course compared with either Ii p31 or wild-type mice (Figure 3). In fact, the clinical score of EAE in p41 mice was approximately two times greater than in p31 mice.
Figure 3.
H-2M– and Ii-deficient mice are resistant to EAE. Mean clinical score of mice immunized with (a) 100 μg pMOG35-55 or (b) 100 μg rMOG emulsified in an equal volume of CFA. Mice also received 150 ng PT at the time of immunization and again 48 hours later as described in Methods.
MOG p35-55 primed T cells from H-2M–deficient, but not Ii-deficient, mice proliferate and secrete Th1 cytokines.
Ii- and H-2M–deficient mouse strains have distinct differences in their CD4+ T cell compartments (15–18). As a result of deficient thymic positive selection, Ii-deficient mice have a reduction of peripheral CD4+ T cells (15, 16). Despite alterations in both class II expression and T cell differentiation, Ii-deficient mice were able to mount a normal Th1 response and control Leishmania major infection in vivo (25). In contrast to Ii-deficient mice, APCs from H-2M–deficient mice express normal class II levels, and H-2M–deficient mice have a broad peripheral CD4+ T cell compartment (17, 18). However, competition with CLIP on I-Ab could prevent effective priming of p35-55–specific Th1 cells in H-2M–deficient mice. Thus, we evaluated whether or not EAE resistance in Ii- and H-2M–deficient mice was due to an inability to prime p35-55–specific Th1 cells or was linked to a defect in their CD4+ T cell compartments. Immunization of Ii-deficient mice with p35-55 did not stimulate a significant proliferative response (Figure 4a) or secretion of IFN-γ (Figure 4b), reflecting an inability to prime p35-55–specific CD4+ T cells. In contrast, T cells from p35-55–immunized H-2M–deficient mice proliferated nearly as well as T cells from wild-type and also secreted substantial IFN-γ. Thus, p35-55 elicited a Th1 response in H-2M–deficient, but not in Ii-deficient, mice. IL-4, a Th2 cytokine, was not detected in cultures from any mice tested (data not shown).
Figure 4.
T lymphocytes from p35-55–immunized H-2M, Ii p31, and Ii p41, but not Ii-deficient, mice proliferate and secrete Th1 cytokines. Ten days after immunization with p35-55, spleen cells were isolated and cultured with various concentrations of p35-55. Proliferative responses are shown in a, and IFN-γ secretion in b. Immunizations, proliferation assays, and cytokine analyses from supernatants were performed as described Methods.
Lymphocytes from EAE-susceptible Ii p31 and p41 mice were also examined (Figure 4). Even though T cells from p35-55–immunized Ii p31 and p41 mice secreted less IL-2 than T cells from wild-type immunized mice, these IL-2 quantities were sufficient to support vigorous proliferation (data not shown). T cells from p35-55–immunized Ii p41 mice secreted similar levels of IFN-γ as T cells from wild-type mice, although T cells from p35-55–immunized p31 mice required nearly one log greater concentration of p35-55 to reach maximal detectable IFN-γ secretion.
Adoptive transfer of encephalitogenic MOG p35-55–specific T cells does not induce EAE in Ii-deficient or H-2M–deficient mice.
As CLIP has a relatively high affinity for I-Ab (26), and it may be necessary for p35-55 to displace CLIP from class II molecules on H-2M–deficient APCs (17, 18), lack of EAE susceptibility in H-2M–recipient mice could reflect an inability of certain APCs to display sufficient p35-55–I-Ab complexes for adequate T cell activation. In contrast to H-2M–deficient APCs, Ii-deficient APCs express “empty” class II molecules (15, 16), and although Ii-deficient APCs may express fewer class II molecules, these APCs present p35-55 to encephalitogenic T cells as well as wild-type APCs (Figure 1).
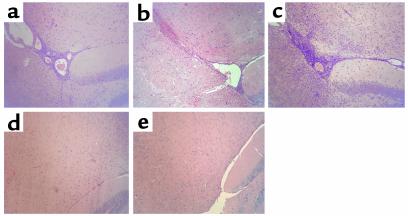
To distinguish the influence of defects in Ag processing and presentation by APCs from alterations in selection of an encephalitogenic T cell repertoire, we examined whether activated wild-type encephalitogenic T cells could induce EAE when adoptively transferred into naive Ii- or H-2M–deficient mice. In contrast to wild-type recipient mice, H-2M– and Ii-deficient–recipient mice did not develop clinical EAE (Table 2) or histological signs of EAE (Figure 5).
Table 2.
Adoptive transfer of encephalitogenic MOG35-55–specific T cells causes EAE in wild-type mice but not Ii-deficient or H-2M–deficient mice
Figure 5.
Histological evidence of EAE in wild-type, Ii p31, and Ii p41, but not Ii- and H-2M–deficient, mice. Inflammatory lesions were observed in (a) wild-type, (b) Ii p31, and (c) Ii p41 mice. No evidence of inflammatory cell infiltration was seen in (d) Ii-deficient or (e) H-2M–deficient mice. CNS tissue was harvested, fixed, and stained with hematoxylin and eosin (H&E) as described in Methods. ×10.
As we observed that H-2M deficient APCs could present p35-55 to encephalitogenic T cells (Figure 1) and immunization of H-2M–deficient mice with p35-55 elicited proliferation and IFN-γ secretion (Figure 4), the reciprocal adoptive transfer using T cells from p35-55–immunized H-2M–deficient mice was performed. We observed that p35-55–specific cells isolated from H-2M–deficient mice induced EAE in wild-type, but not H-2M–deficient, recipient mice (Table 2). Although it is possible that Ii-deficient CNS APCs could not express sufficient MHC class II molecules, our results from H-2M–deficient mice indicate that it was the defect(s) in Ag processing, not an inability to present peptide Ag for T cell activation, that was responsible for the lack of EAE induction in H2-M–deficient mice. In contrast, lack of EAE in Ii-deficient mice reflected both a defect in Ag processing (Figure 1) and an inability to prime (Figure 3) an encephalitogenic p35-55–specific T cell repertoire.
Using a panel of Vβ-specific antibodies (Vβ 4, 5, 6, 8, 12, 13, 14, and 17) for FACS analysis, we examined the TCR repertoire of p35-55–specific encephalitogenic CD4+ T cells isolated from H-2M–deficient and wild-type mice. As reported previously (27), we observed that the repertoire of p35-55–specific T cells in wild-type C57BL/6 mice was heterogeneous (data not shown). In addition, there was no significant difference in usage of TCR Vβ 4,5,6,8,12,13 and 14 between wild-type and H-2M–deficient mice. Only one difference in TCR Vβ usage by p35-55–specific T cells was observed: Vβ 17 was used by 5.3% of wild-type T cells and 10.2% of the H-2M–deficient T cells. Thus, our results demonstrate that inability to induce EAE in H-2M–deficient mice reflects a defect in APC function and not development of a repertoire of encephalitogenic T cells.
Discussion
Endocytic processing may be required at different stages in EAE pathogenesis. When exposed to native MOG outside the CNS, we have observed that processing by peripheral APCs is required for presentation and activation of p35-55–specific T cells. Transmigration across the blood brain barrier (BBB) does not require Ag presentation, as activated T cells, independent of their specificity, can enter the CNS (3, 4). However, recognition of CNS autoantigen is considered a prerequisite for the initiation of T cell–mediated CNS inflammation (3, 4, 28). How then are pathogenic epitopes of CNS autoantigen initially exposed to encephalitogenic Th1 cells? Is endocytic processing required at this stage? Processing independent presentation of CNS autoantigen has been described but is considered rare (29). During turnover of myelin in normal CNS, it is possible that myelin peptides could become accessible to APCs (30). Activated Th1 cells entering the CNS secrete IFN-γ, which causes class II upregulation on resident nonprofessional APCs. Thus if a sufficient pool of myelin peptides exists, autopathogenic epitopes of myelin proteins could be presented to CD4+ Th1 cells by resident CNS APCs. In this situation, endocytic processing may not be required for initial CNS Ag presentation. Alternatively, in normal CNS, a sufficient pool of encephalitogenic protein fragments may not exist or may not be accessible to CNS APC. Through secretion of proinflammatory cytokines, activated Th1 cells that penetrate the BBB may elicit changes that stimulate APCs to upregulate their endocytic machinery, enabling generation and presentation of encephalitogenic peptide products in association with class II molecules. In this regard, microglia (31, 32) and IFN-γ–stimulated astrocytes express cathepsin (Cat) S (J.C. Patarroyo and S.S. Zamvil, unpublished observation), a lysosomal cysteine protease that participates in endocytic processing (33) and MBP degradation (34). Our data in this report are consistent with this latter possibility. Ii-deficient or H-2M–deficient recipient mice did not develop EAE after adoptive transfer of activated wild-type or H-2M–deficient donor encephalitogenic Th1 cells, a situation that obviates the requirement for Ag presentation and T cell activation outside the CNS (2). Thus, even with a repertoire of encephalitogenic T cells, mice containing APCs with defects in Ag processing could not propagate an encephalitogenic CNS inflammatory response, although APCs from these mice could present encephalitogenic MOG peptide to autopathogenic CD4+ T cells.
Alternative splicing of Ii mRNA leads to the formation of p31 and p41 Ii isoforms (9). Ii p41 contains an additional exon, 6b, which encodes a 64–amino acid sequence that is located in the lumenal Ii domain (9). Although controversial (21, 22, 35), several studies have indicated that the less abundant Ii p41 facilitates enhanced class II–restricted presentation of certain Ag’s and antigenic peptide fragments (10, 19, 36). Ii p41 may protect a subset of antigenic epitopes from excessive degradation by inhibiting the lysosomal cysteine protease, Cat L (11, 12). In contrast, more recent evidence indicates that p41 facilitates the maturation of Cat L in selected cell types and prevents its premature destruction by surrounding endocytic proteases (37). Despite differences, p31 and p41 both participate in endocytic localization and class II compact dimer formation, and mice genetically manipulated to express either p31 or p41 support thymic selection of CD4+ T cells and the development of a normal T cell repertoire (21, 22, 35). Although some studies have indicated that Ii p41 may enhance Ag presentation (10–12, 19, 36), this possibility had not been examined in vivo in an organ-specific autoimmune disease. We observed that the incidence of EAE was similar in Ii p31, Ii p41, and wild-type mice, although the disease course was more severe in Ii p41 mice. We also observed that there was a lower threshold for IFN-γ secretion by activated p35-55–specific T cells from Ii p41 mice, which could contribute to the disparity in disease course. Although multiple discrete encephalitogenic determinants have been identified for other CNS autoantigens (38) and repertoire diversification can occur after the initial phase of EAE (5), p35-55 is the only encephalitogenic region of MOG identified in C57BL/6 mice (23, 27). As p41 may confer selective advantage in presentation, one possibility is that p41 facilitates presentation of a cryptic MOG determinant(s), which could participate in the encephalitogenic T cell response in the later phase of acute EAE. We are currently investigating this possibility.
Several proteases that participate in the endocytic pathway have been identified (33). Although it is not clear whether different proteases are utilized for presentation of distinct T cell epitopes, strategies that alter expression of individual proteases may be useful for modifying Ag presentation and T cell activation in clinical diseases (33). In this regard, it was reported that APCs from mice deficient in Cat S have defects in Ag processing and were less susceptible to collagen-induced arthritis, an autoimmune disease mediated by pathogenic Th1 cells (39). In vivo administration of a cysteine protease inhibitor was also effective in preventing Ag presentation to T cells in a model of pulmonary hypersensitivity (40). Similarly, reagents that selectively interfere with the endocytic pathway may be applicable to treatment of CNS autoimmune inflammatory conditions, such as EAE or MS.
Acknowledgments
We thank C. Genain, H. Chapman, P. Nelson, and C.G. Fathman for helpful discussions. We also thank Luc Van Kaer for kindly providing H-2Ma–deficient mice. Support for this work was provided to S.S. Zamvil by the Alexander M. and June L. Maisin Foundation (grant no. 98-416), the Hellman Family Foundation, the NIH (grant no. K02 NS02207), the National Multiple Sclerosis Society (RG 3206-A-3), and the Nancy Davis Center Without Walls. A.J. Slavin and J.M. Soos were supported by postdoctoral fellowships from the National Multiple Sclerosis Society.
Footnotes
Anthony J. Slavin’s present address is: Department of Medicine, Stanford University, Stanford, California, USA.
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 3.Wekerle H, Linington C, Lassman H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 4.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann P, Forsthuber T, Miller A, Sercarz E. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RH. T lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- 7.Soos JM, et al. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol. 1998;161:5959–5966. [PubMed] [Google Scholar]
- 8.Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Immunol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 9.Koch N, Lauer W, Habicht J, Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii): an alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987;6:1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson M, Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature. 1992;357:596–598. doi: 10.1038/357596a0. [DOI] [PubMed] [Google Scholar]
- 11.Bevec T, Stoka V, Pungercic G, Dolenc I, Turk V. Major histocompatibility complex class II-associated p41 invariant chain fragment is a strong inhibitor of lysosomal cathepsin L. J Exp Med. 1996;183:1331–1338. doi: 10.1084/jem.183.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fineschi B, Sakaguchi K, Appella E, Miller J. The proteolytic environment involved in MHC class II-restricted antigen presentation can be modulated by the p41 form of invariant chain. J Immunol. 1996;157:3211–3215. [PubMed] [Google Scholar]
- 13.Rudensky AY, Preston-Hurlburt P, Hong S-C, Barlow A, Janeway CAJ. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;358:764–768. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 14.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 15.Bikoff EK, et al. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viville S, et al. Mice lacking the MHC class II-associated invariant chain. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, et al. Mice lacking H-2M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 18.Martin WD, et al. H-2M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 19.Bikoff EK, Kenty G, Van Kaer L. Distinct peptide loading pathways for MHC class II molecules associated with alternative Ii chain isoforms. J Immunol. 1998;160:3101–3110. [PubMed] [Google Scholar]
- 20.Felix NJ, et al. H2-DMalpha(–/–) mice show the importance of major histocompatibility complex-bound peptide in cardiac allograft rejection. J Exp Med. 2000;192:31–40. doi: 10.1084/jem.192.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaesu NT, Lower JA, Robertson EJ, Bikoff EK. Major histocompatibility class II peptide occupancy, antigen presentation, and CD4+ T cell function in mice lacking the p41 isoform of invariant chain. Immunity. 1995;3:385–396. doi: 10.1016/1074-7613(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 22.Takaesu NT, Lower JA, Yelon D, Robertson EJ, Bikoff EK. In vivo functions mediated by the p41 isoform of the MHC class II-associated invariant chain. J Immunol. 1997;158:187–199. [PubMed] [Google Scholar]
- 23.Slavin A, et al. Induction of a multiple sclerosis-like disease in mice with an immunodominant epitope of myelin oligodendrocyte glycoprotein. Autoimmunity. 1998;28:109–120. doi: 10.3109/08916939809003872. [DOI] [PubMed] [Google Scholar]
- 24.Cho S, Attaya M, Brown MG, Monaco JJ. A cluster of transcribed sequences between the Pb and Ob genes of the murine major histocompatibility complex. Proc Natl Acad Sci USA. 1991;88:5197–5201. doi: 10.1073/pnas.88.12.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DR, et al. T helper subset differentiation in the absence of invariant chain. J Exp Med. 1997;185:31–41. doi: 10.1084/jem.185.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf PR, et al. The phenotype of H-2M-deficient mice is dependent on the MHC class II molecules expressed. Eur J Immunol. 1998;28:2605–2618. doi: 10.1002/(SICI)1521-4141(199809)28:09<2605::AID-IMMU2605>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Mendel Kerlero de Rosbo N, Ben-Nun A. Delineation of the minimal encephalitogenic epitope within the immunodominant region of myelin oligodendrocyte glycoprotein: diverse V beta gene usage by T cells recognizing the core epitope encephalitogenic for T cell receptor V beta b and T cell receptor V beta a H-2b mice. Eur J Immunol. 1996;26:2470–2479. doi: 10.1002/eji.1830261030. [DOI] [PubMed] [Google Scholar]
- 28.Krakowski ML, Owens T. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol. 2000;30:1002–1009. doi: 10.1002/(SICI)1521-4141(200004)30:4<1002::AID-IMMU1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Vergelli M, et al. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur J Immunol. 1997;27:941–951. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- 30.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. I. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- 31.Petanceska S, Canoll P, Devi LA. Expression of rat cathepsin S in phagocytic cells. J Biol Chem. 1996;271:4403–4409. doi: 10.1074/jbc.271.8.4403. [DOI] [PubMed] [Google Scholar]
- 32.Liuzzo JP, Petanceska SS, Moscatelli D, Devi LA. Inflammatory mediators regulate cathepsin S in macrophages and microglia: a role in attenuating heparan sulfate interactions. Mol Med. 1999;5:320–333. [PMC free article] [PubMed] [Google Scholar]
- 33.Villadangos JA, Ploegh HL. Proteolysis in MHC class II antigen presentation: who’s in charge? Immunity. 2000;12:233–239. doi: 10.1016/s1074-7613(00)80176-4. [DOI] [PubMed] [Google Scholar]
- 34.Liuzzo JP, Petanceska SS, Devi LA. Neurotrophic factors regulate cathepsin S in macrophages and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Mol Med. 1999;5:334–343. [PMC free article] [PubMed] [Google Scholar]
- 35.Shachar I, Elliot EA, Chasnoff B, Grewal IS, Flavell RA. Reconstitution of invariant chain function in transgenic mice in vivo by individual p31 and p41 isoforms. Immunity. 1995;3:373–383. doi: 10.1016/1074-7613(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 36.Fineschi B, Arneson LS, Naujokas MF, Miller J. Proteolysis of major histocompatibility complex class II-associated invariant chain is regulated by the alternatively spliced gene product, p41. Proc Natl Acad Sci USA. 1995;92:10257–10261. doi: 10.1073/pnas.92.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lennon-Dumenil A-M, et al. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J. 2001;20:4055–4064. doi: 10.1093/emboj/20.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamvil SS, et al. Multiple discrete encephalitogenic epitopes of the autoantigen myelin basic protein include a determinant for I-E class II restricted T cells. J Exp Med. 1988;168:1181–1186. doi: 10.1084/jem.168.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa TY, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–217. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 40.Riese RJ, et al. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101:2351–2363. doi: 10.1172/JCI1158. [DOI] [PMC free article] [PubMed] [Google Scholar]