Abstract
Objectives
Pulmonary fibrosis is a leading cause of death in systemic sclerosis (SSc). This report examines the differences at baseline and over 12 months between patients with limited versus diffuse cutaneous SSc who participated in the Scleroderma Lung Study.
Methods
SSc patients (64 limited; 94 diffuse) exhibiting dyspnoea on exertion, restrictive pulmonary function and evidence of alveolitis on bronchoalveolar lavage and/or high‐resolution computed tomography (HRCT) were randomised to receive cyclophosphamide (CYC) or placebo and serially evaluated over 12 months.
Results
Baseline measures of alveolitis, dyspnoea and pulmonary function were similar in limited and diffuse SSc. However, differences were noted with respect to HRCT‐scored fibrosis (worse in limited SSc), and to functional activity, quality of life, skin and musculoskeletal manifestations (worse in diffuse SSc) (p<0.05). When adjusted for the baseline level of fibrosis, both groups responded similarly to CYC with regard to lung function and dyspnoea (p<0.05). Cyclophosphamide was also associated with more improvement in skin score in the diffuse disease group more than in the limited disease group (p<0.05).
Conclusions
After adjusting for the severity of fibrosis at baseline, CYC slowed the decline of lung volumes and improved dyspnoea equally in the limited and the diffuse SSc groups. On the other hand, diffuse SSc patients responded better than limited patients with respect to improvements in skin thickening.
Diffuse and limited cutaneous systemic sclerosis (SSc) are differentiated not only by the extent and degree of skin thickening, but also by different propensities to visceral involvement.1,2,3 Patients with diffuse cutaneous SSc (dcSSc) are at a much higher risk for the development of severe heart and kidney involvement than are those with limited cutaneous SSc (lcSSc).2,3 However, data from Steen et al4 suggest that patients with lcSSc are at only a slightly lower risk for the development of severe interstitial lung involvement than are patients with dcSSc. While several studies,5,6,7,8,9 including a recent large randomised placebo‐controlled trial,10 have shown a beneficial effect of cyclophosphamide (CYC) on the course of SSc‐related interstitial lung disease, no study has examined patients with limited versus diffuse SSc for differences in the presentation of their interstitial lung disease or how their pulmonary and systemic manifestations respond to CYC.
The Scleroderma Lung Study (SLS) was a randomised, double‐blind, placebo‐controlled, multi‐center study undertaken to test the hypothesis that early active alveolitis in SSc is amenable to immunosuppressive therapy.10 CYC reduced the decline in pulmonary function and improved dyspnoea, skin thickness, physical function and quality of life over the 12 months of active treatment. This report examines the baseline characteristics of the 64 limited and 94 diffuse SSc patients who entered the SLS, showing equal pulmonary responses but different musculoskeletal, functional and cutaneous responses in the two groups.
Another recent study of cyclophosphamide (given monthly intravenously) confirmed the improvement in lung physiology seen in the cyclophosphamide arm of the SLS but did not address the effect of cyclophosphamide on other aspects of SSc, such as quality of life, function, and skin thickening.11
Materials and methods
Patient selection
The full inclusion and exclusion criteria have been previously published.10 Briefly, patients had to have: SSc as defined by the American College of Rheumatology classification criteria,1 disease onset within the past 7 years (from the first sign or symptom typical of SSc other than Raynaud's), forced vital capacity (FVC) ⩽85% predicted, dyspnoea on exertion ⩾grade 2 on the magnitude of task component of the Mahler Baseline Dyspnoea Index (BDI)12 and alveolitis. Alveolitis was determined by the presence of any ground glass opacification (any GGO, opacification through which lung architecture could be seen) on a high‐resolution computed tomography (HRCT) of the chest and/or by a right middle lobe bronchoalveolar lavage (BAL) that revealed ⩾3.0% neutrophils and/or ⩾2.0% eosinophils when a minimum of 400 cells were counted. In this study, patients were considered to have lcSSc if their skin thickening was confined to areas of the extremities below the elbows and knees and above the clavicles, while patients were considered to have dcSSc if their skin thickening involved the proximal extremities and/or the torso (2).
Exclusion criteria included: FVC <45% of predicted or single‐breath diffusing capacity of the lung for carbon monoxide (DLCO) <30% of predicted; persistent unexplained haematuria: creatinine >2.0 mg/dl; persistent leucopenia or thrombocytopenia; pregnancy or breastfeeding; prior use of oral CYC orally for >4 weeks or >2 intravenous infusions; severe pulmonary hypertension requiring drug therapy; uncontrolled congestive heart failure; smoking during the previous 6 months; prednisone or equivalent in doses >10 mg per day; clinically significant abnormalities noted on chest x ray or HRCT other than interstitial lung disease; use of an angiotensin converting enzyme inhibitor with a sulfhydryl group; active infection which would leave the patient compromised by CYC therapy; other serious illnesses which could compromise the patient's ability to complete the study; significant obstructive pulmonary disease; inflammatory myositis. Willing participants fulfilling all eligibility criteria were randomised on a 1:1 basis to receive either oral CYC in a target dose of 2 mg/kg or matching placebo. All patients provided written informed consent, and the study was approved by the medical institutional review board at each clinical center.
Outcome measurements
Full descriptions of the study outcome measurements have been previously published and are briefly summarised here.10 Pulmonary function tests including DLCO, FVC, forced expiratory volume in the first second (FEV1), total lung capacity (TLC) and the ratio of the residual volume (RV) to TLC (RV/TLC) were performed according to American Thoracic Society Criteria and represented as a percentage of standardised age and height‐adjusted predicted values.13,14,15,16,17,18,19,20,21 Right middle lobe BAL was performed during an outpatient bronchoscopy by serially instilling and recovering four 60‐ml aliquots of room temperature saline according to a predefined protocol.10,22 BAL aliquots from each subject were pooled and used to prepare cytocentrifuge slides that were forwarded to a central core laboratory for the determination of cell differentials by experienced readers. High‐resolution computed tomography of the chest was done according to a predefined procedure and scored in a uniform semiquantitative manner by a single reader.10,23 Other tests included: chest radiograph, serum chemistries, serum creatinine, 24‐h urine for protein and creatinine plus urinalysis with microscopic, modified Rodnan skin score, creatine phosphokinase, joint tenderness and swelling counts, tendon friction rub counts, weight, pulse, respirations, blood pressure, Mahler BDI and the transitional dyspnoea index (TDI) at follow‐up, Health Assessment Questionnaire disability index (HAQ‐DI) plus six visual analogue scales (VAS) examining pain, vascular problems, Raynaud's problems, GI symptoms, breathing symptoms and overall disease severity (called the Scleroderma Health Assessment Questionnaire, SHAQ), 36‐Item Short Form Health (SF‐36) questionnaire and Cough Index.24,25,26
Serial monitoring
All baseline measurements were repeated at 3, 6, 9 and 12 months except for the TDI, which was done only at 6 and 12 months.
Statistical analyses
Descriptive analysis
For each model or quantitative variable, summary statistics were obtained, including mean, standard deviation (SD), median, inter‐quartile difference, minimum and maximum values. Selected correlation coefficients were computed using Kendall's Tau.
Inferential analysis
Several stepwise multiple regression analyses were constructed to determine whether the distribution of cutaneous thickening (limited versus diffuse) was predictive of the following independent variables at baseline: (1) FVC % predicted; (2) DLCO % predicted; (3) dyspnoea (Mahler BDI and breathing VAS individually); (4) HAQ‐DI; and (5) patient global assessment (PGA by VAS). Analysis was also carried out, correlating manual muscle testing with limited versus diffuse SSc skin thickening.
The changes in variables over 12 months in CYC‐treated patients were compared with the changes in the placebo group, for both the limited and the diffuse SSc subsets, by regression analyses. The placebo‐corrected differences (the differences in change scores between the CYC and the placebo groups) in participants with dcSSc were compared with the placebo‐corrected changes in participants with lcSSc using t tests and Wilcoxon tests. When indicated, changes from baseline to follow‐up at 3, 6, 9 and 12 months for indicated parameters were adjusted for the baseline fibrosis score (worst score) on HRCT using a multivariate approach; differences in the time trends between groups were evaluated using a linear splines technique. For patients whose last completed visit was at 6 or 9 months following randomisation, 12‐month data were imputed using a generalised estimating equation (GEE) regression model as recently described.27
There were no adjustments required for multiple comparisons for the variables in table 1. For the outcomes listed in table 2, we applied the well‐known permutation‐based p value adjustment separately for lung physiology, questionnaires, musculoskeletal, and skin outcomes. For the outcomes listed in table 3, we used the same approach to p value and adjustment as in table 2, separately for lung physiology, SF‐36 and other groupings of the outcome variables.
Table 1 Demographic characteristics of patients enrolled in the SLS.
| Variables | Total patients | Limited SSc | Diffuse SSc | p value |
|---|---|---|---|---|
| (n = 158) | (n = 64) | (n = 94) | ||
| Limited/diffuse (%) | 40/60 | |||
| Gender (% female/% male) | 70/30 | 76/24 | 65/35 | 0.16 |
| Age, year (SD) | 48 (13) | 52 (11) | 49 (14) | 0.67 |
| Duration of SSc at entry, year (SD) | 3.1 (2.1) | 3.1 (2.2) | 3.1 (2.0) | 0.83 |
| Duration of Raynaud's at entry, year (SD) | 4.9 (5.2) | 6.4 (6.4) | 3.9 (3.9) | 0.003 |
| Race | ||||
| Caucasian (%) | 68 | 70 | 66 | 0.18 |
| Afro‐American (%) | 16 | 11 | 20 | |
| Other | 16 | 19 | 14 | |
| BAL and HRCT | ||||
| BAL (% alveolitis positive) | 63 | 69 | 60 | 0.60 |
| HRCT (% any GGO) | 91 | 92 | 89 | 0.60 |
Table 2 Baseline disease measures in patients with limited versus diffuse SSc.
| Variable | Total patients | Limited SSc | Diffuse SSc | p value |
|---|---|---|---|---|
| (n = 158) | (n = 64) | (n = 94) | ||
| General | ||||
| Weight (kg) | 72 (7) | 70 (17) | 73 (16) | 0.23 |
| Lung | ||||
| Forced vital capacity (% predicted) | 66.1 (12.1) | 69.3 (12.8) | 67.3 (11.7) | 0.32 |
| Forced expiratory volume‐1 s (% predicted) | 68.9 (12.8) | 70.8 (14.5) | 67.6 (11.4) | 0.13 |
| FEV/FVC ratio (% predicted) | 82.8 (8.0) | 83.6 (6.0) | 82.2 (9.1) | 0.27 |
| Total lung capacity (% predicted) | 69.6 (13.0) | 70.0 (13.4) | 69.5 (12.9) | 0.96 |
| Diffusing capacity (% predicted) | 47.2 (14.0) | 45.7 (13.5) | 48.3 (14.2) | 0.25 |
| Mahler Baseline Dyspnoea Index (0–12) | 5.7 (1.9) | 5.7 (1.5) | 5. 7 (2.1) | 0.89 |
| Breathing VAS (0–100 mm) | 28.4 (26.2) | 25.2 (21.0) | 30.5 (29.0) | 0.22 |
| Cough index (0–4) | 1.9 (0.9) | 2.2 (0.8) | 1.9 (0.8) | 0.06 |
| HRCT and BAL | ||||
| Fibrosis score (worst) | 1.98 (1.04) | 2.09 (0.57) | 1.77 (1.05) | 0.08 |
| Fibrosis score (total) | 7.08 (4.04) | 7.74 (4.07) | 6.31 (3.93) | 0.046 |
| BAL neutrophils (% of total cells) | 6.29 (7.31) | 7.23 (8.11) | 5.62 (6.66) | 0.20 |
| BAL eosinophils (% of total cells) | 2.87 (4.35) | 3.19 (4.69) | 2.65 (4.12) | 0.47 |
| Questionnaires | ||||
| SF‐36, Physical component scale (0–100) | 33.5 (10.8) | 36.0 (10.2) | 31.8 (10.9) | 0.016 |
| SF‐36, Mental component scale (0–100) | 49.5 (10.6) | 51.6 (9.8) | 48.5 (11.0) | 0.08 |
| Disability Index of HAQ (0–3) | 0.8 (0.7) | 0.5 (0.5) | 1.1 (0.7) | 0.0001 |
| Patient global assessment VAS (0–100 mm) | 40.8 (26.6) | 31.6 (24.2) | 47.1 (26.4) | 0.0002 |
| Raynaud's VAS (0–100 mm) | 25.7 (29.0) | 21.2 (26.3) | 28.8 (30.4) | 0.10 |
| GI VAS (0–100 mm) | 13.1 (20.2) | 12.9 (21.3) | 13.3 (19.5) | 0.90 |
| Digital ulcer VAS (0–100 mm) | 13.6 (25.4) | 6.3 (15.1) | 18.5 (29.6) | 0.003 |
| Skin | ||||
| Modified Rodnan skin score (0–51) | 14.6 (10.8) | 5.6 (3.3) | 20.8 (9.8) | 0.0001 |
| Handspread (mm): right | 176.6 (33) | 188.6 (26.4) | 168.3 (34.6) | 0.0003 |
| Musculoskeletal | ||||
| CPK (ratio of observed/upper limit normal) | 1.43 (1.7) | 1.3 (1.8) | 1.5 (1.7) | 0.49 |
| Joint tenderness count (0–8) | 1.0 (2.0) | 0.7 (1.8) | 1.2 (2.2) | 0.18 |
| Joint swelling count (0–8) | 0.6 (1.5) | 0.3 (1.3) | 0.9 (1.6) | 0.06 |
| Proximal muscle weakness (% of subjects) | 11 | 3.7 | 15.9 | 0.03 |
| Tendon friction rubs (% of subjects) | 12 | 0 | 23.2 | 0.0001 |
| Renal | ||||
| Serum creatinine (mg/dl) | 0.75 (0.24) | 0.71 (0.16) | 0.78 (0.27) | 0.11 |
| 24‐h urine protein (mg/24 h) | 0.15 (0.12) | 0.13 (0.13) | 0.15 (0.12) | 0.50 |
Table 3 Changes in outcome measures over 12 months for limited and diffuse SSc patients by treatment group.
| Limited | Diffuse | Diffuse vs Limited | |||||
|---|---|---|---|---|---|---|---|
| CYC | Placebo | CYC | Placebo | CYC | |||
| n = 27 | n = 28 | p values* | n = 46 | n = 44 | p values* | p values** | |
| Pulmonary function | |||||||
| FVC (% predicted) | −0.17 (1.47) | −3.49 (0.95) | 0.03 | −0.35 (0.98) | −1.8 (1.20) | 0.39 | 0.26 |
| TLC (% predicted) | −1.08 (1.77) | −4.43 (1.48) | 0.13 | −0.08 (1.22) | −1.84 (1.25) | 0.40 | 0.30 |
| DLCO (% predicted) | −6.74 (1.59) | −1.47 (1.71) | 0.04 | −2.73 (1.03) | −3.25 (1.26) | 0.70 | 0.82 |
| FEV1 (% predicted) | −0.09 (1.69) | −4.16 (1.29) | 0.05 | −0.29 (0.91) | −2.25 (1.40) | 0.24 | 0.38 |
| TDI (units) | 1.76 (4.03) | −2.64 (3.01) | <0.001 | 1.18 (3.13) | −0.63 (3.28) | 0.02 | 0.03 |
| SF‐36 components | |||||||
| Vitality | 0.46 (3.82) | −7.57 (4.22) | 0.17 | 10.3 (2.41) | 0.44 (2.74) | 0.02 | 0.07 |
| Mental health | 2.76 (2.86) | −3.22 (3.79) | 0.31 | 7.57 (2.10) | 0.69 (2.25) | 0.03 | 0.24 |
| Physical function | 2.46 (4.97) | −7.83 (6.32) | 0.14 | 3.02 (2.72) | −4.80 (2.77) | 0.04 | 0.51 |
| Mental component | 0.83 (1.97) | 1.12 (1.69) | 0.69 | 6.23 (1.05) | 1.57 (1.20) | 0.05 | 0.39 |
| Health transition | −0.78 (0.25) | 0.01 (0.20) | 0.006 | −0.97 (0.2) | −0.36 (0.19) | 0.03 | 0.13 |
| Other outcomes | |||||||
| Skin score (units) | −2.92 (0.94) | −1.33 (1.02) | 0.16 | −5.18 (1.07) | −1.70 (1.42) | 0.03 | 0.001 |
| HAQ−DI | −0.21 (0.08) | 0.05 (0.07) | 0.13 | −0.09 (0.08) | 0.14 (0.08) | 0.04 | 0.646 |
*p values for change from baseline, comparing CYC to placebo patients within limited group and within the diffuse group analysed separately; **p values for comparison of placebo‐corrected change scores in the diffuse group compared to the placebo‐corrected changes scores in the limited group.
Results
Baseline demographic characteristics of the study population as a whole
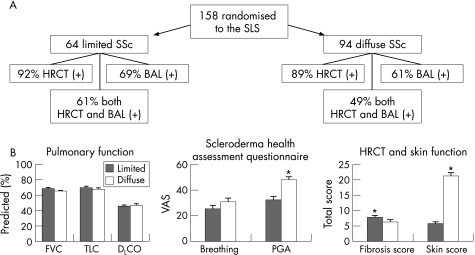
A total of 158 patients met all study criteria and were randomised into the SLS, including 111 females and 47 males. The mean age was 48 (13) years. Forty per cent (64/158) had lcSSc, and 60% (94/158) had dcSSc (table 1, fig 1). The duration of Raynaud's phenomenon in the limited SSc group was significantly longer than in the diffuse SSc group (p = 0.003) at baseline. There were no other important demographic differences noted between limited and diffuse SSc patients at baseline.
Figure 1 Comparison of baseline features: Limited vs Diffuse SSc. (A) 158 patients with SSc, symptomatic restrictive lung disease, and evidence of active alveolitis on either BAL (neutrophilia >3% and/or eosinophilia >2%) and/or HRCT (presence of any ground‐glass opacity) were randomised to the SLS with the breakdown of limited and diffuse SSc, and their findings on BAL and HRCT as shown. (B) Differences between limited and diffuse patients with respect to baseline measures of pulmonary function (FVC, TLC, DLCO), represented as a percentage of predicted normal values; specific components of health related quality of life (Breathing Symptoms Visual Analog Scale (Breathing) and Patient's Global Assessment Visual Analog Scale (PGA) of the Scleroderma Health Assessment Questionnaire), on a 0–100 scale where higher values indicate worse symptoms; and total score for lung fibrosis by HRCT (Fibrosis Score) and modified Rodnan Skin Score (Skin Score), where a higher score indicates more extensive findings. Values represent mean±SE for each group. *p<0.0002 comparing lcSSc with dcSSc.
Comparison of limited and diffuse SSc patients at baseline
There were no significant differences between lcSSc and dcSSc patients related to the frequency of alveolitis by HRCT and/or BAL criteria, the percentage neutrophils or eosinophils in BAL, or the average values for FVC, TLC or DLCO (tables 1 and 2). In addition, a regression analysis failed to show any relationship between the extent or degree of skin thickening (limited, diffuse) or duration of SSc at entry and the FVC % predicted, DLCO % predicted or dyspnoea (either the BDI or breathing VAS) at baseline (p>0.05). The study population as a whole had significant impairments in lung function (table 2).
The baseline HRCT fibrosis score (total) was significantly higher (worse) in patients with lcSSc (p = 0.046). In contrast, the mean modified Rodnan skin score, mean right handspread and the mean swollen joint count were more abnormal in the diffuse group (fig 1). Tendon friction rubs were seen only in patients with dcSSc.
Significant but relatively small differences in many of the questionnaire responses were noted between the lcSSc and dcSSc groups (table 2), including the mean physical component score (PCS) of the SF‐36, the mean HAQ‐DI, and the mean digital ulcer VAS, all of which were more abnormal in the diffuse group. The patient global assessment (PGA) VAS was markedly worse in the diffuse than the limited group (47.1 (26.4) vs 31.6 (24.2), respectively; p<0.001). The mean Raynaud's VAS and the mean GI‐VAS were not significantly different between groups.
Comparison of the course of limited and diffuse SSc patients over 12 months
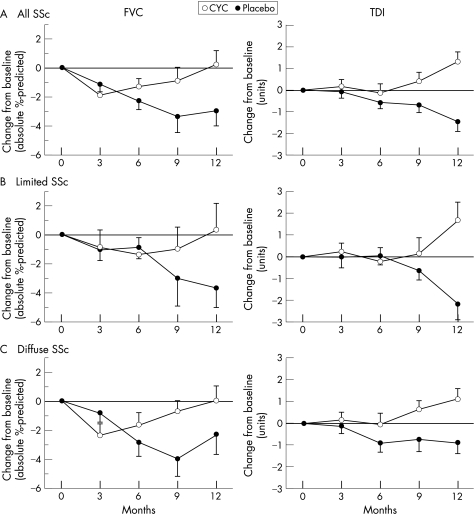
While differences in the course of pulmonary function, dyspnoea and health‐related quality of life (HRQoL) appeared greater in the limited placebo patients compared with the diffuse placebo SSc patients (table 3), these differences disappeared after adjusting for the maximal fibrosis score measured on the baseline HRCT (fig 2).
Figure 2 Changes over 12 months in patients on Placebo vs CYC. The adjusted change from baseline for the FVC (absolute percentage predicted) and for the TDI (total score in Units) were calculated for patients in the Placebo and CYC treatment groups for all individuals in the study (A, n = 66 for CYC, 64 for Placebo), those with Limited SSc (B, n = 26 for CYC, 26 for Placebo), and patients with Diffuse SSc (C, n = 40 for CYC, 38 for Placebo) using a multivariate model accounting for differences in the Maximal Fibrosis Score on HRCT as measured at baseline. Median (FVC) and mean (TDI) values±SE.
Compared with placebo‐treated patients as a whole, those treated with CYC experienced smaller declines in FVC and TLC, and actually reported improvements in dyspnoea. As in the placebo group, there were no differences in response to CYC comparing patients with lcSSC and dcSSc with respect to FVC, TLC or TDI once the results were adjusted for maximum fibrosis scores on baseline HRCT. The apparent differences between limited and diffuse patients with respect to progression of their pulmonary disease and the response to CYC were related primarily to differences in their pulmonary disease status at the time of presentation (more fibrosis in the limited group).
There were few differences between groups with regard to extra‐pulmonary variables. After adjusting for the placebo responses, for example, dcSSc patients responded better than lcSSc patients with respect to improvement in skin score. Other aspects of HRQoL (health transition, mental component summary score, and physical function component of the SF‐36 and the HAQ‐DI) changed equally in response to CYC for both lcSSc and dcSSc patients.
Discussion
As recently reported by the SLS,10 treatment with CYC for 12 months was associated with an overall slowing in the decline of lung volumes (FVC % predicted and TLC % predicted) and an improvement in dyspnoea, skin thickening (skin score), functional ability (HAQ‐DI) and several components of HRQoL (SF‐36) when compared with placebo in a randomised, double‐blinded, clinical trial. The current analysis focuses on differences between patients with limited versus diffuse cutaneous SSc, both at presentation to the study and over the 1 year of treatment with either CYC or placebo. Since dcSSc patients have a greater degree of skin involvement and have a predilection for a much higher incidence of renal and cardiac involvement than is seen in lcSSc patients,1,2,3 the assumption has been that SSc‐related ILD would be more common and severe in patients with diffuse SSc. However, as observed in other studies,4,5 approximately 40% of the patients enrolled into the SLS with dyspnoea, restrictive lung disease and evidence of alveolar inflammation on BAL or HRCT had lcSSc. It is clear from these data that all patients with SSc, regardless of their characterisation as either lcSSc or dcSSc, warrant careful evaluation for the presence of lung involvement. However, the SLS was not designed to evaluate the prevalence of lung disease in SSc, and so a careful characterisation of the frequency and severity of lung involvement in these two patient populations remains to be carried out. For example, available data suggest that there are twice as many patients with limited SSc as there are with diffuse SSc.28,29 As such, even though patients with lcSSc represented 40% of our study patients, the frequency of lung disease in this population as a whole might still be considerably less than that in patients with dcSSc.
In the SLS, patients with limited and diffuse SSc were virtually identical with respect to age, time between onset of SSc and presentation, baseline description of their dyspnoea, frequency of positive BAL and/or HRCT findings, and baseline pulmonary function (table 1). However, some differences were noted. Patients with lcSSc were observed to have more severe fibrosis on HRCT and longer duration of Raynaud's phenomenon. As would be expected, patients with dcSSc exhibited more skin abnormalities (higher modified Rodnan skin scores, smaller handspread, and more abnormal digital ulcer VAS) and musculoskeletal problems (proximal muscle weakness and tendon friction rubs). Consistent with these disease manifestations, they also complained of greater impairments in function and HRQoL (lower PCS of the SF‐36, higher HAQ‐DI scores, and a more abnormal PGA VAS).
A multivariate analysis from the SLS identified baseline FVC and fibrosis score as the most important independent predictors of the decline over time in pulmonary function.10 While limited and diffuse patients were indistinguishable with respect to their baseline pulmonary function, patients with limited SSc presented with more extensive fibrosis on HRCT and therefore appeared to experience a more rapid decline in pulmonary function and dyspnoea. There may be several explanations for the apparent difference in fibrosis at presentation. It may be more difficult to date the onset of disease in limited patients, resulting in a lead‐time bias. However, if this were the case, pulmonary function tests should have also been worse. Given the smaller sample size associated with a sub‐analysis and the multiple endpoints that were examined, this difference could have also been by chance. Finally, it is possible that limited and diffuse patients exhibit slightly different patterns of pulmonary disease, similar to the differences in the distribution and severity of their skin disease. Despite these apparent baseline differences between lcSSc and dcSSc, patients with lcSSc and dcSSc experience a similar decline in pulmonary function (FVC, TLC) and worsening of dyspnoea over time, when adjusted for the level of fibrosis on HRCT. An appropriately powered prospective analysis will be required to resolve whether more extensive fibrosis at baseline is a true characteristic of limited SSc.
In contrast to the respiratory findings and their changes over time, our study supports the observations of others,2,3 that abnormalities in skin and musculoskeletal systems, as well as functional ability and quality of life, are much more pronounced in early dcSSc than in early lcSSc. Significantly, the SLS is the first study to demonstrate that CYC has a significant positive effect on skin thickening in dcSSc. Patients with dcSSc present with more severe skin and musculoskeletal involvement, which may explain the greater abnormality in HRQoL in this subgroup. With more involvement at baseline, it might be expected that greater improvements would be seen in skin, musculoskeletal and HRQoL in dcSSc patients treated with CYC.
In summary, treatment with CYC slowed the rate of decline of lung volumes and improved dyspnoea in patients with SSc‐related interstitial lung disease regardless of whether they presented with limited or diffuse cutaneous manifestations. Most dcSSc patients already undergo careful evaluation for ILD. This study suggests that patients with lcSSc (which made up 40% of our patient population) should also undergo careful evaluation and treatment for ILD. In addition, lcSSc and dcSSc patients presented with identical abnormalities in pulmonary function and dyspnoea, suggesting that ILD may be just as severe in this group as it is in those with diffuse disease. On the other hand, the lcSSc patients in the SLS study population had more extensive fibrosis on HRCT scans at baseline. Whether or not lcSSc patients, in general, actually have more extensive fibrosis and therefore are at a greater risk for progressing without treatment remains to be clarified. Finally, treatment with CYC had a greater positive impact on skin and musculoskeletal involvement in dcSSc patients, resulting in more substantial improvements in their health‐related quality of life than that observed in lcSSc patients. Future trials of possible disease‐modifying therapy should take the unique characteristics of lcSSc and dcSSc SSc into account with respect to both study design and analysis.
Acknowledgements
Supported by a grant from the Public Health Service (UO1 HL60587 to the UCLA Clinical Center) and by grants from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to the NHLBI). Grant support for the other clinical centers and the Data Coordinating Center is listed below. We are grateful to Bristol‐Myers Squibb for kindly supplying cyclophosphamide at no charge.
Abbreviations
BAL - bronchoalveolar lavage
CYC - cyclophosphamide
dcSSC - diffuse cutaneous systemic sclerosis
DLCO - single‐breath diffusing capacity of the lung for carbon monoxide
FEV1 - forced expiratory volume in the first second
FVC - forced vital capacity
GEE - generalised estimating equation
GGO - ground glass opacification
HAQ‐DI - Health Assessment Questionnaire disability index
HRCT - high‐resolution computed tomography
HRQoL - health‐related quality of life
lcSSC - limited cutaneous systemic sclerosis
PGA - patient global assessment
RV - residual volume
SD - standard deviation
SF‐36 - 36‐Item Short Form Health
SHAQ - Scleroderma Health Assessment Questionnaire
SLS - Scleroderma Lung Study
SSc - systemic sclerosis
TDI - transitional dyspnoea index
TLC - total lung capacity
VAS - visual analogue scales
Footnotes
Competing interests: None of the investigators has any financial relationship with Bristol‐Myers Squibb.
References
- 1.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 198023581–590. [DOI] [PubMed] [Google Scholar]
- 2.Clements P J, Medsger T A., Jr Cutaneous involvement. In Clements PJ, Furst DE, eds. Systemic sclerosis. Philadelphia: Lippincott Williams & Wilkins 2004129–150.
- 3.Steen V D, Medsger T A., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000432437–2444. [DOI] [PubMed] [Google Scholar]
- 4.Steen V D, Conte C, Owens G R, Medsger T A., Jr Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994371283–1289. [DOI] [PubMed] [Google Scholar]
- 5.White B, Moore W C, Wigley F M, Xiao H Q, Wise R A. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med 2000132947–954. [DOI] [PubMed] [Google Scholar]
- 6.Latsi P I, Wells A U. Evaluation and management of alveolitis and interstitial lung disease in scleroderma. Curr Opin Rheumatol 200315748–755. [DOI] [PubMed] [Google Scholar]
- 7.Silver R M, Warrick J H, Kinsella M B, Staudt L S, Baumann M H, Strange C. Cyclophosphamide and low‐dose prednisone therapy in patients with systemic sclerosis (scleroderma) with interstitial lung disease. J Rheumatol 199320838–844. [PubMed] [Google Scholar]
- 8.Akesson A, Scheja A, Lundin A, Wollheim F A. Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum 199437729–735. [DOI] [PubMed] [Google Scholar]
- 9.Giacomelli R, Valentini G, Salsano F, Cipriani P, Sambo P, Conforti M L.et al Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol 200229731–736. [PubMed] [Google Scholar]
- 10.Tashkin D P, Elashoff R, Clements P J, Goldin J, Roth M D, Furst D E.et al Cyclophosphamide versus placebo in scleroderma lung disease. N Eng J Med 20063542655–2666. [DOI] [PubMed] [Google Scholar]
- 11.Hoyles R K, Ellis R W, Wellsbury J, Lees B, Newlands P, Goh N S, Roberts C, Desai S, Herrick A L, McHugh N J, Foley N M, Pearson S B, Emery P, Veale D J, Denton C P, Wells A U, Black C M, du Bois R M. A multicenter, prospective, randomized, double‐blind, placebo‐controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006543962–3970. [DOI] [PubMed] [Google Scholar]
- 12.Mahler D A, Weinberg D H, Wells C K, Feinstein A R. The measurement of dyspnea: contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 198485751–758. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Standardization of spirometry—1994 update. Am Rev Respir Dis 19951521107–1136. [DOI] [PubMed] [Google Scholar]
- 14.American Association for Respiratory Care Respir Care. 1994;39:1184–1190. [PubMed] [Google Scholar]
- 15.British Thoracic Society Guidelines for the measurement of respiratory function: recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med 199488165–194. [PubMed] [Google Scholar]
- 16.American Thoracic Society Single‐breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 19951522185–2198. [DOI] [PubMed] [Google Scholar]
- 17.Black L F, Hyatt R E. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 196999696–702. [DOI] [PubMed] [Google Scholar]
- 18.Crapo R O, Morris A H, Gardner R M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981123659–664. [DOI] [PubMed] [Google Scholar]
- 19.Crapo R O, Morris A H, Clayton P D, Nixon C R. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 198218419–429. [PubMed] [Google Scholar]
- 20.Crapo R O, Morris A H. Standardized single breath normal values of carbon monoxide diffusing capacity. Am Rev Respir Dis 1981123185–189. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society Lung function testing: Selection of reference values and interpretative strategies. Am Rev Respir Dis 19911441202–1218. [DOI] [PubMed] [Google Scholar]
- 22.Clements P J, Goldin J G, Kleerup E G, Furst D E, Elashoff R M, Tashkin D P.et al Regional differences in bronchoalveolar lavage and thoracic high‐resolution computed tomography in dyspnea patients with systemic sclerosis (scleroderma). Arthritis Rheum 2004501909–1917. [DOI] [PubMed] [Google Scholar]
- 23.Goldin J, Lynch D, Strollo D, Strange C, Silver R, Clements P.et al High resolution CT findings in scleroderma‐related lung diseases. Proceedings of ATS . 2006;3A105
- 24.Steen V D, Medsger T A., Jr The value of the Health Assessment Questionnaire and special patient‐generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997401984–1991. [DOI] [PubMed] [Google Scholar]
- 25.Ware J E, Jr, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 26.Petty T L. The National Mucolytic Study. Results of a randomized, double‐blind, placebo‐controlled study of iodinated glycerol in chronic obstructive bronchitis. Chest 19909875–83. [DOI] [PubMed] [Google Scholar]
- 27.Elashoff R M, Li G, Li N. An approach to joint analysis of longitudinal measurements and competing risks failure time data. Statist Med. In press. [DOI] [PMC free article] [PubMed]
- 28.Mayes M D, Lacey J V, Jr, Beebe‐Dimmer J, Gillespie B W, Cooper B, Laing T J.et al Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003482246–2255. [DOI] [PubMed] [Google Scholar]
- 29.Le Guern V, Mahr A, Mouthon L, Jeanneret D, Carzon M, Guillevin L. Prevalence of systemic sclerosis in a French multi‐ethnic county. Rheumatology (Oxford) 2004431129–1137. [DOI] [PubMed] [Google Scholar]