Abstract
Aim
In this study, we employed chimeric human/mouse Proteinase 3 (PR3) proteins as tools to induce an autoantibody response to PR3 in rats and mice.
Method
Rats and mice were immunised with recombinant human PR3 (HPR3), recombinant murine PR3 (mPR3), single chimeric human/mouse PR3 (HHm, HmH, mHH, mmH, mHm, Hmm) or pools of chimeric proteins. Antibodies to mPR3 and HPR3 were measured by ELISA. Antibodies to rat PR3 were determined by indirect immunofluorescence (IIF) on rat white blood cells. Urinalysis was performed by dipstick analysis. Kidney and lung tissue was obtained for pathological examination.
Results
In mice, immunisation with the chimeric human/mouse PR3 Hmm led to an autoantibody response to mPR3. Rats immunised with the chimeric human/mouse PR3 Hmm, HmH and mmH, or a pool of the chimeric human/mouse PR3 proteins, produced antibodies selectively binding to rat granulocytes as detected by IIF. No gross pathological abnormalities could be detected in kidney or lungs of mice or rats immunised with chimeric human/mouse PR3.
Conclusion
Immunisation with chimeric human/mouse proteins induces autoantibodies to PR3 in rats and mice. Chimeric proteins can be instrumental in developing experimental models for autoimmune diseases.
Wegener's granulomatosis (WG) is associated with antineutrophil cytoplasmic antibodies (ANCA),1 in particular to Proteinase 3 (PR3).2 PR3‐ANCA are a specific and sensitive marker for WG, whereas other ANCA‐associated vasculitides are associated with antimyeloperoxidase (MPO) antibodies.3 The association between fluctuations in PR3‐ANCA and relapsing disease in WG suggests a pathogenic role for PR3‐ANCA.4 However, although animal models support a pathogenic role for MPO‐ANCA in vasculitis development5 attempts to develop an animal model for PR3‐ANCA‐associated vasculitis have not been successful thus far.6 Recently, chimeric Pr3 proteins that are partly composed of the human amino acid sequence and partly of the sequence of the mouse homologue have been described.7 In this study, we employed these chimeric proteins as immunological tools to induce an autoantibody response in rats and mice to PR3. We hypothesised that, by epitope spreading,8 antibodies could develop to rat or mouse PR3 leading to an autoimmune response to PR3. As the homology between rat and mouse PR3 is 94%, antibodies to mouse PR3 will likely also recognise rat PR3.9
In a set of experiments, rats and mice were immunised with separate chimeric human/mouse PR3 proteins or combinations thereof. Indeed, rats immunised with chimeric human/mouse PR3 developed autoantibodies to mouse PR3 and rat granulocytes and mice immunised with one specific chimeric human/mouse PR3 induced antibodies to mouse PR3. The results provide the first evidence that an autoantibody response can be generated in rats and mice by immunisation with chimeric proteins.
Materials and methods
Animals
Experiments were performed in conventionally housed 10‐week‐old female Wistar Kyoto (WKY) rats, Brown Norway (BN) rats and C57BL/6J mice (Harlan, Bilthoven, The Netherlands). Experiments were approved by the local animal care and experimentation committee.
Immunisation
Animals were immunised with recombinant human PR3 (HPR3), recombinant mouse PR3 (mPR3) and chimeric human/mouse PR3 proteins. The latter were generated by the double overhang splicing PCR technique using the mPR3 and HPR3 cDNA as templates.7 The chimeric constructs were named according to the origin of the respective portions of the molecule, where H denotes HPR3 and m denotes mPR3. Six chimeric proteins (HHm, HmH, mHH, mmH, mHm and Hmm) were generated. All recombinant molecules were generated as enzymatically inactive proteins by substitution of the active site Ser to Gly.
In a first set of experiments, WKY rats and C57BL/6J mice were primed by intraperitoneal injection of 10 μg of HPR3, mPR3, HHm, HmH, mHH, mmH, mHm or Hmm in Complete Freund's Adjuvant (CFA) supplemented with H37Ra (Difco, Detroit, MI). On day 21, animals received a boost immunisation intraperitoneally with 10 μg of protein in incomplete Freund's Adjuvant (Difco). At 0, 3, 6 and 8 weeks, serum samples were collected. At weeks 3 and 7, animals were placed in metabolic cages for 16 h to obtain urine samples. Animals were sacrificed at 8 weeks, and kidneys and lungs were collected.
In a second set of experiments, WKY and BN rats were primed by intraperitoneal injection with a pool of the six chimeric human/mouse PR3 proteins (10 μg of each protein) or 60 μg bovine serum albumin (BSA, Sigma Chemicals, St. Louis, MO) in CFA supplemented with H37Ra. Rats received boost immunisations at 3, 5 and 7 weeks intraperitoneally with a pool of the chimeric human/mouse PR3 or BSA in iCFA. At 0, 5, 7, 9, 10 and 21 weeks, serum samples were collected. Animals were sacrificed at 10 weeks, and kidneys, lungs and urine were collected. An overview of the experimental set‐up is given in table 1.
Table 1 Set‐up of the experimental groups.
| Proteins (amount) used for immunisation | Rat strain (no. of animals) | Mouse strain (no. of animals) | No. of immunisations | LPS (mg/kg) |
|---|---|---|---|---|
| HPR3 (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| mPR3 (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| HHm (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| HmH (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| mHH (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| mmH (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| mHm (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| Hmm (10 μg) | WKY (4) | C57BL/6J (4) | 2 | – |
| Pool human/mouse PR3 (60 μg) | WKY (3)/BN (3) | – | 4 | – |
| Pool human/mouse PR3 (60 μg) | WKY (3)/BN (3) | – | 4 | 1 |
| Pool human/mouse PR3 (60 μg) | WKY (3)/BN (3) | – | 4 | 2.5 |
| BSA (60 μg) | WKY (3)/BN (3) | – | 4 | – |
| BSA (60 μg) | WKY (3)/BN (3) | – | 4 | 1 |
| BSA (60 μg) | WKY (3)/BN (3) | – | 4 | 2.5 |
Detection of antimouse and antihuman PR3 antibodies
Antibodies specific for mPR3 or HPR3 were detected by anti‐PR3 ELISA as described previously.7 Dilutions of rat or mouse serum ranged from 1:20 to 1:12 500. Alkaline‐phosphatase labelled rabbit antirat IgG or sheep antimouse IgG (Sigma) was used as conjugate.
Detection of antigranulocyte antibodies
Rat leucocytes were fixed on glass slides by means of ethanol, preincubated with 1% NGS and incubated with rat sera (1:10 starting dilution) for 60 min at room temperature. Bound antibodies were detected by FITC‐conjugated goat antirat IgG (Southern Biotechnology Associates, Birmingham, AL). Nuclei were counterstained with 10 ng/ml of diamidino phenyl indole (Sigma).
Statistical analysis
All results are expressed as the mean ± SEM and were analysed using the two‐tailed Mann–Whitney U test.
Results
Mice immunised with chimeric human/mouse PR3 produce autoantibodies to mouse PR3
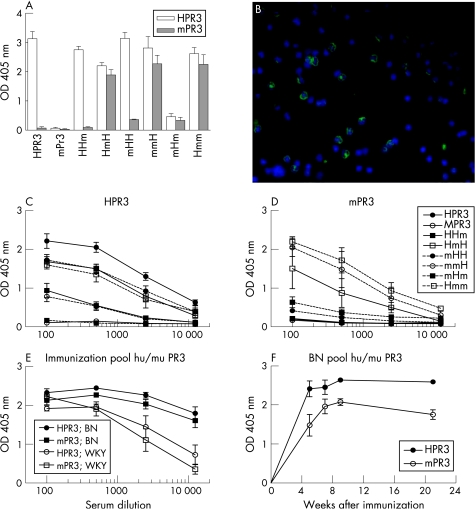
Immunisation of mice with HPR3 induced an antibody response to HPR3 without any cross‐reactivity to mPR3 being observed. Immunisation with mPR3 did not induce an antibody response to mPR3 or HPR3 (fig 1A). Mice immunised with the chimeric proteins HHm, HmH, mHH and Hmm produced antibodies to HPR3 (fig 1B), whereas only immunisation with the chimeric protein Hmm induced antibodies to mPR3 (fig 1C). No antibodies to HPR3 or mPR3 were detected in serum samples obtained before immunisation
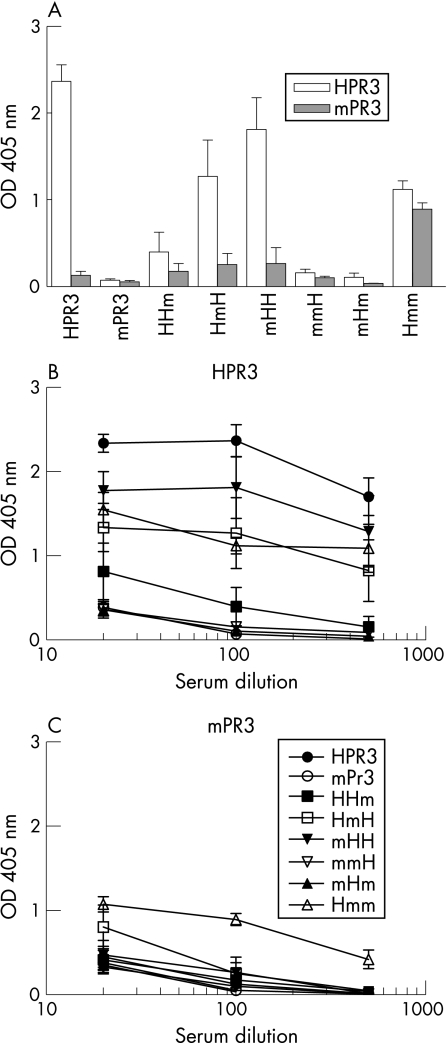
Figure 1 Antibodies to human PR3 (HPR3) and mouse PR3 (mPR3) in sera of mice immunised with HPR3, mPR3 and human/mouse PR3 chimeric proteins. (A) Antibodies to HPR3 (white bar) and mPR3 (grey bar) were determined by direct ELISA in 1:100 diluted sera of mice immunised with different chimeric proteins 8 weeks after the first immunisation. Antibodies to HPR3 (B) and mPR3 (C) were determined by direct ELISA in 1:20 to 1:500 diluted sera of mice immunised with different chimeric proteins 8 weeks after the first immunisation. Means with SEM values are shown four 4 mice per group.
Rats immunised with chimeric human/mouse PR3 produce autoantibodies to mouse PR3 and rat granulocytes
Immunisation of rats with HPR3 induced an antibody response to HPR3 but not to mPR3. Immunisation with mPR3 did not induce an antibody response to mPR3 or HPR3 (fig 2A). Rats immunised with chimeric human/mouse PR3 produced high titre antibodies to HPR3, except for rats immunised with mHm (fig 2C). Immunisation with Hmm, HmH and mmH also induced antibodies to mPR3 with titres comparable with those observed for antibodies to HPR3 (fig 2D).
Figure 2 Antibodies to human PR3 (HPR3), mouse PR3 (mPR3) and rat granulocytes in sera of rats immunised with HPR3, mPR3 and human/mouse PR3 chimeric proteins. (A) Antibodies to HPR3 (white bar) and mPR3 (grey bar) were determined by direct ELISA in 1:100 diluted sera of rats immunised with different chimeric proteins 8 weeks after the first immunisation. Antibodies to rat granulocytes in serum 8 weeks after the first immunisation (B) of rats immunised with HPR3, mPR3 and human/mouse PR3 chimeric proteins (in this figure immunised with the chimeric protein HmH) were determined by indirect immunofluorescence on ethanol‐fixed rat white blood cells. The staining is shown with nuclear staining (blue). Sera of rats immunised with human/mouse PR3 chimeric proteins only recognised rat granulocytes (green) and not lymphocytes. The serum dilution was 1:50. Antibodies to HPR3 (C) and mPR3 (D) were determined by direct ELISA in 1:100 to 1:12 500 diluted sera of rats immunised with different chimeric proteins 8 weeks after the first immunisation. Means with SEM values are shown for 4 rats per group. Antibodies to HPR3 and mPR3 (E) were determined by direct ELISA in 1:100 to 1:12 500 diluted sera of rats (WKY or BN rats) immunised with a pool of 6 chimeric proteins 10 weeks after the first immunisation. Antibodies to HPR3 and mPR3 (F) were determined by direct ELISA in 1:640 diluted sera of rats (WKY or BN rats) immunised with a pool of 6 chimeric proteins up to 21 weeks after the first immunisation. Means with SEM values are shown for 3 rats per group.
Higher titres of antibodies to HPR3 and mPR3, still present after 21 weeks, were induced in BN rats compared with WKY rats after immunisations with a pool of chimeric human/mouse PR3 (fig 2E,F). Sera recognising mPR3 also bound selectively to granulocytes of rats (fig 2B). Before immunisation, no antibodies to HPR3, mPR3 or rat granulocytes were detected
Autoantibodies to mouse or rat PR3 do not induce autoimmune disease
In the kidney and lungs of mice or rats immunised with either HPR3, mPR3, single chimeric human/mouse PR3 or pools of chimeric proteins, no gross pathological abnormalities could be detected. In all groups of mice or rats, urinalysis by dipstick revealed no abnormalities
Discussion
Here, we demonstrate that chimeric proteins can be used to induce an autoantibody response to autologous PR3 in rats and mice. Despite high titre autoantibodies, no vasculitis‐associated lesions were observed.
No cross‐reactivity to rat or mPR3 was seen after immunisation with HPR3, a phenomenon that has been described for MPO,10 suggesting that the homology of 69% between HPR3 and the rat (GenBank accession AF503440) or mouse homologue9 is too low for cross‐reactivity to occur.
In both species, however, immunisation with chimeric human/mouse PR3 did induce an antibody response to autologous PR3. So, the initial recognition of the “foreign” human PR3 part was essential for spreading to recognition of autologous PR3 via intramolecular epitope spreading. As the protein sequence of rat PR3 (GenBank accession AF503440) and mPR3 share 94% of their amino acids,9 it is likely that the antigenic specificity of the antibodies binding to mouse and rat PR3 are the same. Numerous in vitro and in vivo data support a direct pathogenic role of ANCA in systemic vasculitis (reviewed in Heeringa et al11),5 but a convincing PR3‐ANCA‐associated animal model has not yet been established.6 To further investigate the pathogenic role of autoantibodies to PR3, we performed a pilot experiment in which purified IgG from rats immunised with a pool of chimeric human/mouse PR3 proteins or BSA was injected into C57bl/6 mice (2 mg/kg body weight). After 6 days, mice were sacrificed. No significant urinary abnormalities were observed; nor did we find any pathological alterations in kidneys and lungs. Thus, high titre autoantibodies to PR3 alone appear insufficient in inducing disease manifestations.
The mechanisms accounting for the apparent differences in pathogenicity between PR3‐ANCA and MPO‐ANCA in animal models are unclear, but several possibilities may be considered.
First, it was reported that the functional characteristics of mPR3 are more similar to human and murine neutrophil elastase than to HPR312 suggesting that, under inflammatory conditions, mPR3 will not be expressed on the membrane of neutrophils. Membrane expression of HPR3 on neutrophils plays a key role in the pathophysiology of ANCA‐associated vasculitis.13 Thus, human and rats/mice differ too much, at least with respect to the functional properties and membrane expression of PR3, which makes appropriate interpretation of the experiments performed on the pathogenic role of anti‐PR3 autoantibodies in rats and mice difficult. It is thus premature to suggest that autoantibodies to PR3 alone are insufficient in inducing vasculitis in humans.
Second, differences in ionic strength between mMPO, HPR3 and mPR39,14) could explain differences in tissue retention, which could account for the discrepancies in pathophysiological effects of autoantibodies to MPO and PR3 in experimental models.
In conclusion, autoantibodies to autologous PR3 can be induced in mice and rats by immunisation with chimeric human/mouse PR3 proteinsbut do not induce vasculitis. The use of chimeric proteins could provide a new way to study breakdown of tolerance to other self‐antigens.
Acknowledgements
This study was supported by a grant from the Dutch Kidney Foundation. P Heeringa is supported by a grant from the Dutch Organisation of Scientific Research (NWO VIDI 917.66.341). The authors would like to thank H van der Molen and AJ Petersen, for their excellent support with animal experiments. The generation of chimeric human/mouse PR3 proteins was supported by a grant from the Swedish research council (grants 71X‐15152 and 73X‐09487).
Abbreviations
ANCA - antineutrophil cytoplasmic antibodies
BN - Brown Norway
BSA - bovine serum albumin
HPR3 - human proteinase 3
MPO - myeloperoxidase
mPR3 - murine proteinase 3
PR3 - proteinase 3
WG - Wegener's granulomatosis
WKY - Wistar Kyoto
Footnotes
Competing interests: None declared.
References
- 1.van der Woude F J, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es L A.et al Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 19851425–429. [DOI] [PubMed] [Google Scholar]
- 2.Goldschmeding R, van der Schoot C E, ten Bokkel Huinink D, Hack C E, van den Ende M E, Kallenberg C G M.et al Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate‐binding protein in the lysosomes of normal human neutrophils. J Clin Invest 1989841577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk R J, Jennette J C. Anti‐neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 19883181651–1657. [DOI] [PubMed] [Google Scholar]
- 4.Cohen Tervaert J W, Huitema M G, Hene R J, Sluiter W J, The T H, van der Hem G K.et al Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet 1990336709–711. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y.et al Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002110955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister H, Ollert M, Frohlich L F, Quintanilla‐Martinez L, Colby T V, Specks U.et al Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood 20041041411–1418. [DOI] [PubMed] [Google Scholar]
- 7.Selga D, Segelmark M, Wieslander J, Gunnarsson L, Hellmark T. Epitope mapping of anti‐PR3 antibodies using chimeric human/mouse PR3 recombinant proteins. Clin Exp Immunol 2004135164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderlugt C L, Miller S D. Epitope spreading in immune‐mediated diseases: implications for immunotherapy. Nat Rev Immunol 2002285–95. [DOI] [PubMed] [Google Scholar]
- 9.Jenne D E, Frohlich L, Hummel A M, Specks U. Cloning and functional expression of the murine homologue of proteinase 3: implications for the design of murine models of vasculitis. FEBS Lett 1997408187–190. [DOI] [PubMed] [Google Scholar]
- 10.Little M A, Smyth C L, Yadav R, Ambrose L, Cook H T, Nourshargh S.et al Anti‐neutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte–microvascular interactions in vivo. Blood 20051062050–2058. [DOI] [PubMed] [Google Scholar]
- 11.Heeringa P, Huugen D, Tervaert J W. Anti‐neutrophil cytoplasmic autoantibodies and leukocyte–endothelial interactions: a sticky connection? Trends Immunol 200526561–564. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner O, Litwiller R D, Hummel A M, Viss M A, McDonald C J, Jenne D E.et al Differences between human proteinase 3 and neutrophil elastase and their murine homologues are relevant for murine model experiments. FEBS Lett 20055795305–5312. [DOI] [PubMed] [Google Scholar]
- 13.Rarok A A, Stegeman C A, Limburg P C, Kallenberg C G M. Neutrophil membrane expression of proteinase 3 (PR3) is related to relapse in PR3‐ANCA‐associated vasculitis. J Am Soc Nephrol 200212232–2238. [DOI] [PubMed] [Google Scholar]
- 14.Kao R C, Wehner N G, Skubitz K M, Gray B H, Hoidal J R. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest 1988821963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]