We report a 74‐year‐old man with acute on chronic tophaceous gout in whom conventional treatments failed but who responded to treatment with the interleukin‐1 receptor antagonist, anakinra. The patient presented in February 2004 with a severe flare of gout. Multiple joints were swollen, including the right fifth proximal interphalangeal (PIP), the left fourth PIP, and the first metatarsophalangeal (MTP) joints bilaterally. Apart from chronic tophaceous gout, he also had a history of membranous glomerulonephritis (for which he was on prednisolone 5 mg/day), hypertension, and ischaemic heart disease. Allopurinol had previously induced a severe anaphylactic reaction and his renal impairment was exacerbated by non‐steroidal anti‐inflammatory drugs.
On examination, the above mentioned joints were swollen with associated tenderness and erythema, and there were multiple tophi. Investigations revealed a raised C reactive protein (CRP) of 72 mg/l (normal <10 mg/l), a raised urate of 0.6 mmol/l (0.20–0.42 mmol/l), urea of 13.9 mmol/l, and creatinine of 155 μmol/l, with creatinine clearance reduced to 54 ml/min. The patient could only tolerate colchicine 0.5 mg daily and probenicid 1 g daily, both of which were continued throughout anakinra therapy. A prednisolone dose of up to 40 mg daily was used but his symptoms worsened whenever steroids were tapered. This inadequate control resulted in foot deformities with dropping of the right metatarsal arch, associated with radiographic osteopenia and erosions of the left first interphalangeal joint. Febuxostat, benzbromarone, and uricase inhibition were considered but were not used because of lack of availability or concerns about toxicity.1,2,3 However, recent data show that uric acid activates the NALP3 inflammasome, leading to release of interleukin‐1 (IL‐1β), which provides a novel theoretical basis for anti‐IL‐1β treatment.4,5,6,7,8,9
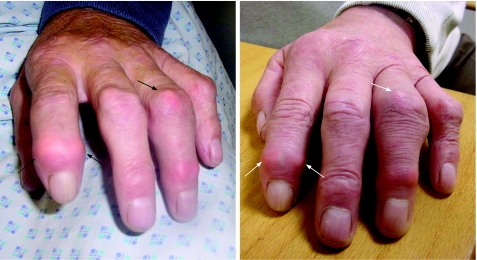
The patient was prescribed anakinra 100 mg daily subcutaneously, and outcomes were assessed regularly thereafter. The joints involved were photographed before and after therapy (fig 1) with the patient's consent. Pre‐therapy, the total swollen joint count (SJC) was 14 and the tender joint count (TJC) 24. The CRP and erythrocyte sedimentation rate (ESR) were normal pre‐therapy (<1 mg/l and 13 mm/h, respectively), probably reflecting the effect of the steroid treatment. The patient improved within two weeks, with the global assessment score falling from 65 mm to 20 mm on a visual analogue scale (VAS 0–100 mm), and SJC improving from 14 to 3. The physician global assessment score fell from 80 mm to 50 mm. The patient received a continuous daily dose of anakinra during the follow up as he had residual joint tenderness, and maintained the benefit from this treatment at six months. Prednisolone 5 mg daily was continued for the glomerulonephritis. We plan to wean the patient off anakinra if symptoms resolve.
Figure 1 Left hand of the patient before treatment with anakinra, and three months after starting anakinra. Acute inflammatory tophi can be seen on the index finger distal interphalangeal joint and the proximal interphalangeal (PIP) joint of the ring finger (black arrows) pre‐therapy. These erythematous and swollen joints improved following treatment with anakinra (white arrows). Note that the angle of the photograph highlights the joint deformity in the PIP joint of the ring finger in the post‐anakinra image, but joint diameters were unchanged from before treatment with anakinra. Images reproduced with the patient's consent.
There is surprisingly little information about the management of “intractable” gout, and treatment options remain empirical. So et al have recently shown the efficacy of anakinra in resistant gout in a small pilot study.9 In contrast to other reports, our patient was at the most severe end of the disease spectrum but nevertheless improved significantly. There has been one report on the successful use of anti‐tumour necrosis factor in severe gout.10 It remains to be determined whether anakinra is especially effective in gout or whether antagonism of other key cytokine pathways is also effective.
References
- 1.Kaufmann P, Torok M, Hanni A, Roberts P, Gasser R, Krahenbuhl S. Mechanisms of benzarone and benzbromarone‐induced hepatic toxicity. Hepatology 200541925–935. [DOI] [PubMed] [Google Scholar]
- 2.Pui C H. Urate oxidase in the prophylaxis or treatment of hyperuricemia: the United States experience. Semin Hematol 200138(4 suppl 10)13–21. [DOI] [PubMed] [Google Scholar]
- 3.Chu R, Lin Y, Reddy K C, Pan J, Rao M S, Reddy J K.et al Transformation of epithelial cells stably transfected with H2O2‐generating peroxisomal urate oxidase. Cancer Res 1996564846–4852. [PubMed] [Google Scholar]
- 4.Akahoshi T, Murakami Y, Kitasato H. Recent advances in crystal‐induced acute inflammation [review]. Curr Opin Rheumatol 200719146–150. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A.et al Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature 200640237–241. [DOI] [PubMed] [Google Scholar]
- 6.Murakami Y, Akahoshi T, Hayashi I, Endo H, Hashimoto A, Kono S.et al Inhibition of monosodium urate monohydrate crystal‐induced acute inflammation by retrovirally transfected prostaglandin D synthase. Arthritis Rheum 2003482931–2941. [DOI] [PubMed] [Google Scholar]
- 7.Roberge C J, Grassi J, De Medicis R, Frobert Y, Lussier A, Naccache P H.et al Crystal‐neutrophil interactions lead to interleukin‐1 synthesis. Agents Actions 19913438–41. [DOI] [PubMed] [Google Scholar]
- 8.Di Giovine F S, Malawista S E, Nuki G, Duff G W. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal‐induced IL 1. J Immunol 19871383213–3218. [PubMed] [Google Scholar]
- 9.So A, Desmedt T, Revaz S, Tschopp J. A pilot study of IL‐1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 10.Tausche A K, Richter K, Grassler A, Hansel S, Roch B, Schroder H E. Severe gouty arthritis refractory to anti‐inflammatory drugs: treatment with anti‐tumour necrosis factor alpha as a new therapeutic option. Ann Rheum Dis 2004631351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]