Abstract
Objectives
(1) To investigate whether inflammatory synovial tissues from patients with rheumatoid arthritis (RA) express endothelial protein C receptor (EPCR) and (2) to determine the major cell type(s) that EPCR is associated with and whether EPCR functions to mediate the effects of activated protein C (APC) on these cells.
Methods
EPCR, CD68 and PC/APC in synovial tissues were detected by immunostaining and in situ PCR. Monocytes were isolated from peripheral blood of patients with RA and treated with APC, lipopolysaccharide (LPS), and/or EPCR blocking antibody RCR252. Cells and supernatants were collected for RT‐PCR, western blotting, enzyme‐linked immuosorbent assay and chemotaxis assay.
Results: EPCR was expressed by both OA and RA synovial tissues but was markedly increased in RA synovium. EPCR was colocalised with PC/APC mostly on CD68 positive cells in synovium. In RA monocytes, APC upregulated EPCR expression and reduced monocyte chemoattractant protein‐1‐induced chemotaxis of monocytes by approximately 50%. APC also completely suppressed LPS‐stimulated NF‐κB activation and attenuated TNF‐α protein by more than 40% in RA monocytes. The inhibitory effects of APC were reversed by RCR252, indicating that EPCR is required.
Conclusions
Our results demonstrate for the first time that EPCR is expressed by synovial tissues, particularly in RA, where it co‐localises with PC/APC on monocytes/macrophages. In addition, APC inhibits the migration and activation of RA monocytes via EPCR. These inhibitory effects on RA monocytes suggest that PC pathway may have a beneficial therapeutic effect in RA.
Rheumatoid arthritis (RA) is a chronic autoimmune disease with persistent inflammation of multiple synovial joints, which results in progressive tissue destruction of bone and cartilage.1,2 It is characterised by the infiltration of inflammatory cells (neutrophils, monocytes and lymphocytes) into the synovial compartment and the production of inflammatory mediators. In RA, monocytes migrate into the synovium to become activated macrophages where they secrete significant amounts of inflammatory cytokines such as interleukin (IL)‐1, tumour necrosis factor (TNF)‐α and proteases, which are important in initiating, propagating and maintaining synovial inflammation.3 Macrophages can also differentiate into dendritic cells and osteoclasts,4 the latter being recognised as the key cellular effectors of pathological bone erosion in arthritis.5 In rheumatoid synovial sections, most synovial lining cells are highly activated macrophage‐like cells functioning as antigen‐processing and antigen‐presenting cells to T lymphocytes.6 Macrophages are critically involved in the pathogenesis of RA, not only by producing a variety of pro‐inflammatory cytokines and chemokines, but also by contributing to the cartilage and bone destruction.
Activated protein C (APC) is a 61‐kDa serine protease derived from its vitamin K‐dependent plasma precursor, protein C (PC). Activation of PC occurs on the endothelial cell surface and is triggered by a complex formed between thrombin and thrombomodulin. The conversion to APC is augmented in the presence of its specific receptor, endothelial protein C receptor (EPCR),7 which is expressed on the surface of endothelial cells, keratinocytes8 and some leucocytes, including eosinophils, neutrophils and monocytes.9
APC acts as an anticoagulant by neutralising the procoagulant activities of factors Va and VIIIa and inhibiting thrombin generation. In addition, APC exerts significant anti‐inflammatory properties, associated with a decrease in pro‐inflammatory mediators and a reduction of leucocyte recruitment.10 Many anti‐inflammatory properties of APC are mediated through EPCR, which itself can independently exert anti‐inflammatory effects.11,12,13 For example, severe EPCR deficiency adversely affects survival and cardiac function of mice subjected to challenge by endotoxin infusion.13 Baboons treated with an antibody to block PC binding to EPCR respond lethally to normally sublethal concentrations of E coli and exhibit disseminated intravascular coagulation, intense neutrophil influx into the tissues and elevation of inflammatory cytokines, indicating that EPCR provides a critical step in the host defense against E coli.12 Over expression of EPCR protects transgenic mice from endotoxin‐induced injury.14 In addition, recent findings suggest that EPCR is required for embryo survival and development.15,16,17
PC/APC is elevated in RA synovial fluid and synovial joints, where it co‐localises with MMP‐2.18 However, whether EPCR is present in the inflammatory joint is unknown. The purpose of this study was: (1) to determine whether inflammatory (RA) synovial tissue expresses EPCR and if so whether these levels are higher than non‐inflammatory OA synovial tissue; and (2) to elucidate the major cell type(s) EPCR is associated with and whether it functions to mediate the effects of APC on these cells.
Materials and methods
Patient population
Whole blood samples (20 ml/patient) were obtained from 19 patients with RA (13 females, mean 50.5 (SD 10.3) years of age; 6 males, mean 54.0 (7.1); synovial tissues (8 RA comprising 7 females, mean 68.2 (5.1) years of age and a 70‐year‐old male; 8 osteoarthritis (OA) comprising 3 females, mean 67.0 (4.6) years of age and 5 males, mean 69.5 (3.6) years of age) were obtained from patients undergoing joint replacement surgery in accordance with approval by the ethics committee of Royal North Shore Hospital. All patients fulfilled the American College of Rheumatology criteria for RA19 and OA,20 and gave their written informed consent.
Monocyte isolation
Whole blood was collected in tubes containing ethylenediaminetetraacetic acid. Monocytes were isolated using Optiprep (Sigma, St. Louis, MO) according to the manufacturer's instructions. The viability of the monocytes was determined by the Trypan Blue exclusion test. The purity of the monocytes was determined by anti‐CD68 antibody (DAKO Corporation, Carpinteria, CA) immunostaining. Cells were used for further experiments if both viability and purity were above 95%.
Cell culture and treatment
Monocytes were suspended into RPMI‐1640 with 2% fetal calf serum (FCS) (basal medium) and treated with recombinant APC (Xigris, Eli Lilly, IN), RCR252 (EPCR blocking antibody), RCR92 (EPCR non‐blocking antibody) (anti‐EPCR antibodies were gifts from Professor Fukudome, Department of Immunology, Saga Medical School, Nabeshima, Saga, Japan) and lipopolysaccharide (LPS; Sigma).
Chemotaxis assay
Monocyte chemotaxis was examined by modified Boyden chambers21 with a 5‐μm‐pore‐size filter. Briefly, 30‐μl aliquots of recombinant monocyte chemoattractant protein (MCP)‐1 (0.01, 0.1, 1 and 10 ng/ml) and medium were added into the lower chamber wells, and aliquots of 50 μl cells (5×105 cells/ml, in RPMI‐1640 with 2% bovine serum albumin with no pretreatment or pretreated with APC or APC in the presence of RCR252 or RCR92) were added to each of the upper wells. The chambers were assembled according to the manufacturer's instructions (Neuro Probe, Gaithersburg, MD) and incubated at 37°C with 5% CO2 for 60 min. After the incubation, the filter was removed, the top of the filter was scraped to remove cells that did not undergo chemotaxis, and then the filter was subjected to Diff‐Quik staining (Baxter, McGraw Park, IL). The number of cells that had migrated through the filter was calculated by counting the total number of cells in 15 separate fields of view under ×40 power. Results are expressed as the number of cells that migrated per high‐power field after subtracting the background (unstimulated control) to demonstrate specific migration.
ELISA
Cells (0.5 ml of 1×106 cells/ml) in RPMI‐1640 with 2% FCS were treated with various test agents for 24 h, Cell supernatants were collected and centrifugated at 10 000 g for 15 min to remove any cell or cell debris. TNF‐α in cell culture supernatants was measured by ELISA kits (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions.
Western blot analysis
Monocytes were washed three times with phosphate‐buffered saline (PBS), and lysis buffer (0.15 M NaCl, 0.01 mM PMSF, 1% NP‐40, 0.02 M Tris and 6 M urea/H2O) was added and incubated for 30 min at 4°C. Cell lysates were centrifuged at 10 000 g for 15 min. Protein concentration in the supernatant was determined using the Bradford protein assay (Bio‐Rad, Hercules, CA). Equally loaded protein from culture supernatants and cell lysates was separated by 10% sodium‐dodecyl‐sulfate polyacrylamide‐gel electrophoresis and electrotransferred onto Immobion™ Transfer membranes (PVDF) (Millipore, Bedford, MA). Membranes were then blocked and incubated with a rabbit antihuman EPCR antibody (Invitrogen, Carlsbad, CA), a mouse antihuman active form of NF‐κB antibody (Chemicon International, Temecula, CA) followed by incubation with horseradish peroxidase‐conjugated secondary antibodies. Immunoreactivity was detected using the ECL detection system (Amersham, Piscataway, NJ). Anti‐β‐actin (Sigma) antibody was also included to adjust the equal loading.
Immunohistochemical staining and analysis
Human synovial tissues (8 OA and 8 RA) were fixed with 10% PBS‐buffered formalin and embedded using paraffin. Tissue sections (4 μm) were de‐paraffinised and processed for immunostaining using goat antihuman EPCR antibody (2 μg/ml, R&D systems). Negative control samples were incubated with the same concentration of goat IgG. After primary antibody incubation, tissues were stained with a DAKO LSAB+Systems stain kit (DAKO Corporation) and counterstained with Haematoxylin and Scotts Blueing Solution.
The stained slides were examined to identify the cellular localisation of EPCR and scored for intensity and proportion of synovial cells by an independent observer who was blinded to the patient characteristics and outcome. EPCR immunoreactivity was scored separately for expression of lining cells, endothelium, sublining in synovial tissues with RA and OA. An EPCR total score was also obtained. The staining intensity was determined in five random areas at 400× magnification, regardless of the percentage of staining. The intensity scores were 0 (no staining), 1 (weak staining), 2 (moderate) and 3 (intense staining). The proportion of stained areas was scored as 0 (absence of immunoreactivity), 1 (<10% positivity), 2 (10–70%) and 3 (70–100%) by reading five random areas at 400× magnification, regardless of the staining intensity. The possible scores were 1 (focal, <10%), 2 (regional, 11–50%), and 3 (diffuse, >50%). A weighted score for each specimen was produced by multiplying the proportion score by the intensity score.
Immunofluorescent staining
For fluorescent staining, de‐paraffinised tissue sections were incubated with a goat antihuman EPCR antibody, a mouse antihuman CD68 antibody (DAKO Corporation) and a rabbit antihuman PC antibody (Sigma). Negative control samples were incubated with the same concentration of goat, mouse and rabbit IgG. After washing with PBS for three times, tissue sections were then incubated with antirabbit IgG conjugated with Cy3 (red), antimouse IgG conjugated with amca (blue) and antigoat IgG conjugated with FITC (green) (1:400, Sigma‐Aldrich) for 2 h. After another three washes, tissue sections were mounted and observed under a fluorescence microscope (Nikon ECLIPSE 80i, Nikon Corporation, Tokyo, Japan). Images were acquired and processed using a Nikon digital camera and software (Diagnostic Instruments, Sterling Heights, MI, USA) and Image J (http://rsb.info.nih.gov/ij).
In situ RT‐PCR
De‐paraffinised RA synovial tissue sections were air‐dried and treated with pepsin (2 mg/ml) for 30 min followed by a 1‐min wash with diethyl pyrocarbonate‐treated water and 1‐min wash with ethanol. Air‐dried tissue was digested with DNAase I (0.2 U/μl; Epicentre, Madison, WI) overnight at 37°C and processed for in situ RT‐PCR with a biotin‐labelled EPCR primer pair (450 bp). EPCR primer sequences were: sense primer 5′ CCTCAGATGGCCTCCAAA3′; antisense primer 5′ AGGCATTGAGCTGCTGCA3′. AMV reverse transcriptase was omitted in negative control samples. After in situ PCR, tissue sections were washed with PBS for 5 min and blocked with 1% H2O2 for 15 min to quench the activity of peroxidase. After 2 washes with PBS, tissue sections were processed for immunostaining using DAKO LSAB+Systems stain kit (DAKO Corporation) and counterstained with Haematoxylin and Scotts Blueing Solution.
Statistical analysis
Significance was determined using one‐way ANOVA followed by a Student–Newman–Keuls test. For analysis of EPCR expression in OA and RA synovial tissues, the Wilcoxon non‐parametric test was used for comparison between OA and RA. Any p values less than 0.05 were considered statistically significant.
Results
EPCR is expressed in synovial membrane and is colocalised with PC/APC
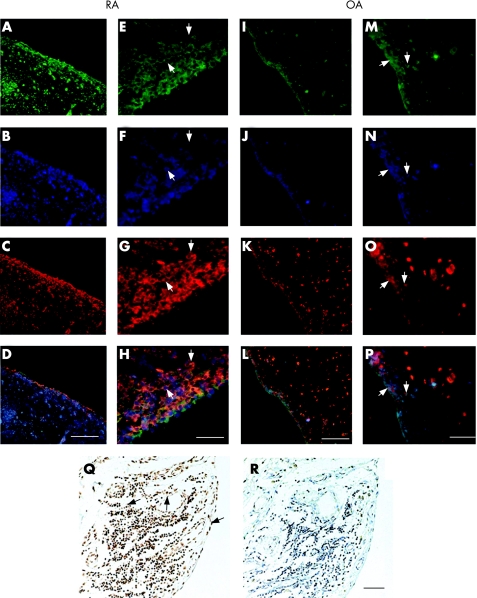
Expression of EPCR was assessed by immunofluorescent and immunohistochemical staining using paraffin‐embedded sections of synovial membranes from 8 patients with RA and 8 patients with OA. In all sections, EPCR was immunolocalised to endothelial cells lining the blood vessels, cells in the synovial lining layer and infiltrating cells in the sub‐lining layer (fig 1A, E, I and M). There was a marked increase in the intensity of staining in the synovial lining cells and infiltrating cells of RA compared with OA synovium (fig 1A, E, I and M). Semiquantative analysis of EPCR expression in synovial tissues showed that mean scores for total EPCR protein were significantly higher in RA synovium than in OA synovium (5.6 vs 3.4, p<0.01, table 1). The scores for EPCR in the lining, sublining and endothelium were also substantially higher in RA synovium compared with OA (table 1).
Figure 1 EPCR expression in RA and OA synovial tissues and its co‐localisation with PC/APC and CD68 positive cells. Confocal images of EPCR (green), CD68 (blue) and PC/APC (red) expression in synovial tissue detected by immunofluorescent staining. D, H, L and P are merged images of A, B and C; E, F and G; I, J and K and M, N and O, respectively. A–H indicate RA synovial tissues; I–P indicate OA synovial tissues. White arrows show examples of cells positively stained for EPCR, CD68 and PC/APC. Scale bar for A–D and I–L: 100 μm. Scale bar for E–H and M–P: 20 μm. In situ RT‐PCR on RA synovial tissue was used to measure EPCR gene expression (Q). Black arrows show examples of positive stained cells. Negative control (R) showed no staining. Scale bar for Q and R: 50 μm.
Table 1 EPCR expression in different regions of synovial tissues of patients with RA and OA.
| Rheumatoid arthritis (n = 8) | Osteoarthritis (n = 8) | p value | |
|---|---|---|---|
| Overall EPCR | 5.6 (1.3) | 3.4 (1.3) | 0.0069) |
| Lining EPCR | 7.8 (1.5) | 5.5 (1.3) | 0.0029) |
| Sublining EPCR | 5.0 (2.02) | 2.4 (1.0) | 0.0019) |
| Endothelial EPCR | 3.8 (1.02) | 2.5 (1.2) | 0.015) |
EPCR immunoreactivity was scored as described in Materials and methods. Results are shown as mean (SD. The non‐parametric Wilcoxon test was used for statistical analysis.
A major proportion of synovial cells are macrophage‐like cells.6 To identify the cellular source of EPCR and to examine whether EPCR is colocalised with its ligand, PC/APC, we performed triple immunofluorescent staining using antibodies to PC/APC and CD68 (monocyte/macrophage marker) in addition to EPCR. Similar to EPCR staining, there was noticeably more intense staining for PC/APC and CD68 in RA synovial tissue compared with OA tissue (fig 1). CD68 was prominently expressed throughout the lining layer and the invading cells in the sublining layer. There was a considerable overlap of staining for all 3 proteins (fig 1D, H, L and P). All CD68 positive cells expressed EPCR, but not all CD68 or EPCR positive cells expressed PC/APC (fig 1).
In order to confirm that EPCR is produced locally in the joint, in situ PCR was employed to detect tissue gene expression. Similar to its protein localisation, EPCR mRNA was strongly expressed by lining cells, endothelium and infiltrating inflammatory cells in the sublining layer (fig 1Q).
APC upregulates the expression of EPCR in RA monocytes
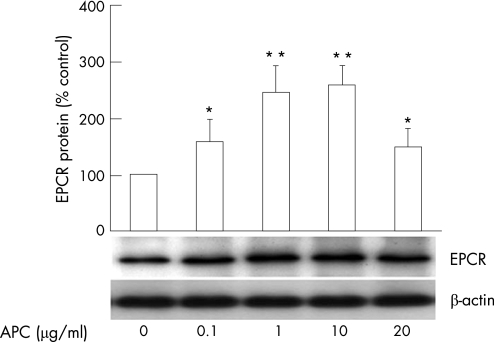
EPCR expression was measured in purified RA monocytes using western blotting. EPCR was expressed by RA monocytes under basal conditions and the addition of APC (1 and10 μg/ml) stimulated protein levels of EPCR more than 2 times higher than the control (fig 2). Consistent with the protein expression, EPCR gene expression by RA monocytes was prominent under basal conditions and upregulated in response to APC (data not shown).
Figure 2 EPCR expression by RA monocytes. Monocytes were isolated from peripheral blood of patients with RA and treated with APC at 0, 0.1, 1, 10 and 20 μg/ml for 4 h. EPCR protein in cell lysates from RA monocytes was detected by western blotting and semiquantified by image analysis relative to β‐actin control. Data are expressed as mean±SEM (n = 3) % of control (no treatment). *p<0.05; **p<0.01.
APC inhibits of the migration of RA monocytes via EPCR
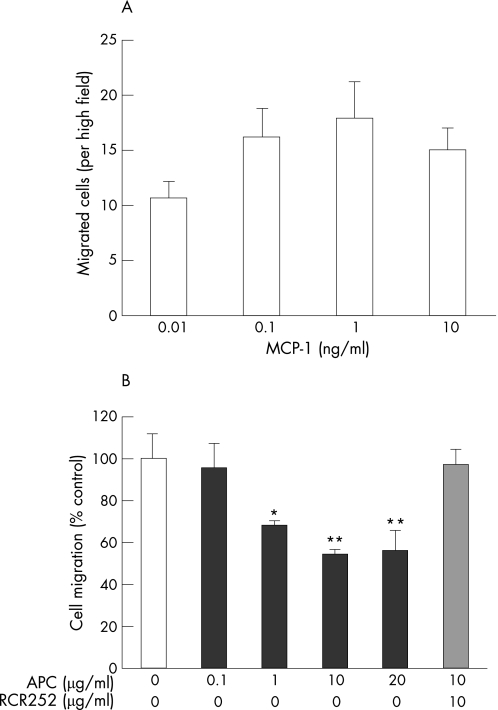
Directed migration of monocytes into the synovium is an important event in the pathogenesis of RA.3 We first tested the dose response of monocyte chemotaxis to MCP‐1, using a modified Boyden chamber assay. At 1 ng/ml, MCP‐1 displayed its maximum stimulatory effect on cell migration (fig 3A). Using this optimal concentration, we next examined the effect of APC on MCP‐1‐stimulated monocyte chemotaxis. When monocytes were pretreated with APC, cell migration toward MCP‐1 was inhibited in a dose‐dependent manner with a maximal inhibitory effect greater than 40% (fig 3B). Preincubation with RCR252 abolished the effect of APC on the cell migration, whereas the non‐blocking control antibody, RCR92, had no significant effect (data not shown). These results indicate that APC acts through EPCR to inhibit the chemotactic response of RA monocytes.
Figure 3 APC inhibits MCP‐1‐induced migration of RA monocytes. (A) Chemotaxis of RA monocytes toward MCP‐1 (0.01, 0.1, 1 and 10 ng/ml) was measured using a modified Boyden chamber. Cell migration is expressed as the average number of cells in 15 separate fields of view under ×40 power that migrated after subtracting the background (unstimulated control migration toward medium). (B) RA monocytes chemotaxis toward MCP‐1 (1 ng/ml) when cells were preincubated with APC (0, 0.1, 1, 10, 20 μg/ml) in the presence or absence of RCR252 (10 μg/ml) for 30 min. Results are shown as a percentage of control cells (without APC treatment) that migrated toward 1 ng/ml MCP‐1. Data are presented as mean (SD). *p<0.05; **p<0.01.
APC acts through EPCR to inhibit NF‐κB and TNF‐α in RA monocytes
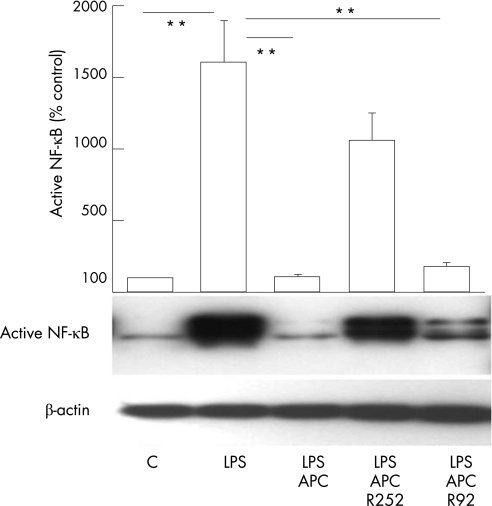
NF‐κB is an important inflammatory signalling pathway and plays a pivotal role in both initiation and perpetuation of chronic inflammation of RA.22,23 To measure NF‐κB, whole cell lysates from LPS‐stimulated RA monocytes were subjected to western blot using an antibody which recognises the active form of NF‐κB. Results are shown in fig 4. The activity of NF‐κB in RA monocytes was increased more than 15‐fold in response to LPS treatment. Addition of APC (20 μg/ml) completely inhibited the LPS‐stimulated increase in NF‐κB. This inhibitory effect of APC on NF‐κB was almost totally reversed by RCR252 (10 μg/ml), whereas RCR92 had no significant effect, indicating that APC acts through EPCR to inhibit NF‐κB.
Figure 4 APC inhibiting the activation of NF‐κB in monocytes. The active form of NF‐κB in whole cell lysates of monocytes was detected by western blotting using an antibody against active NF‐κB. RA monocytes were treated with no test agent (C), LPS (100 ng/ml), LPS plus APC (20 μg/ml) in the presence or absence of RCR252 (R252, 10 μg/ml) or RCR92 (R92, 10 μg/ml). Images were semiquantified using image‐analysis software. Data are expressed as mean (SEM) (n = 3) % of control (no treatment). **p<0.01.
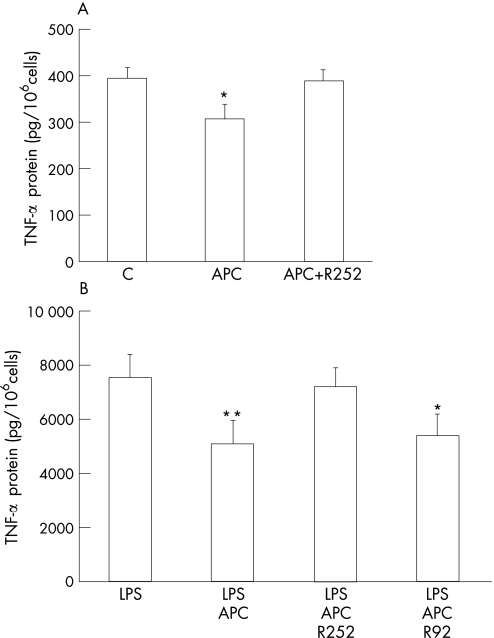
Monocytes/macrophages are the primary source of TNF‐α which, after release, promotes the proinflammatory activity of these and other surrounding cells.24,25 TNF‐α protein levels were detected in RA monocytes by ELISA. The low levels of TNF‐α detected in cell supernatants from RA monocytes under basal conditions (fig 5A) were substantially increased by LPS stimulation (fig 5B). APC significantly decreased TNF‐α by approximately 25% and 40% in control and LPS‐stimulated cells, respectively (fig 5A, B). RCR252 completely reversed the effect of APC, whereas the control antibody RCR92 had no significant effect.
Figure 5 APC inhibits the expression of TNF‐α by monocytes from RA patients. (A) RA monocytes were treated with APC (20 μg/ml), APC plus RCR252 (R252, 10 μg/ml) or no test agent (C) for 24 h. TNF‐α protein levels in culture supernatants were measured by ELISA. (B) RA monocytes were treated with LPS (100 ng/ml), APC (20 μg/ml) plus LPS, APC plus LPS with RCR252 (10 μg/ml) or with RCR92 (R92, 10 μg/ml) for 24 h. TNF‐α protein levels in culture supernatants were detected by ELISA. Data represent mean (SEM) (n = 3). *p<0.05, **p<0.001.
Discussion
This is the first report to show that EPCR is expressed by synovial tissues. In RA synovium, EPCR expression was substantially stronger than OA tissue, particularly in the sublining layer where infiltrating inflammatory cells reside. In agreement with our previous study,18 PC/APC is also elevated in the synovium of patients with RA. Here, we show that EPCR is immunolocalised with PC/APC in synovial tissue.
In addition to EPCR's anti‐inflammatory effects, its crystal structure has recently revealed a potential new role in regulating the immune response.26 EPCR is structurally similar to the CD1/MHC class I family of molecules and can bind a ligand in the lipid‐containing antigen presenting groove. The CD1 family members play direct roles in presenting lipid antigens to T cells and host defence against certain bacterial pathogens.27,28 Together, these data imply that the elevated level of EPCR in RA synovium, found in the current study, may play a potentially protective role in response to the abnormal immune and inflammatory changes in RA.
Immunostaining for CD68 identified the majority of cells expressing EPCR in the lining and sublining layers as monocytes/macrophages (fig 1). These cells were also the major cells associated with PC/APC in synovium. Although monocytes provide beneficial effects during wound healing and chronic bacterial infections, they have been linked to various inflammatory diseases. After activation, monocytes/macrophages secrete inflammatory cytokines/chemokines and proteases which amplify the existing inflammation and exacerbate the disease status. Monocyte/macrophage infiltration in the synovium is a prominent feature in RA,29 and their numbers correlate significantly with the severity of disease.3 Circulating peripheral blood monocytes isolated from RA patients exhibit an activated phenotype closely resembling monocytes in the synovium, such as activation of inflammation‐associated genes, spontaneous production of cytokines, and increased secretion of matrix‐degrading enzymes.3 In the current study, the anti‐inflammatory effects of APC were demonstrated by its ability to reduce the basal production of TNF‐α by RA monocytes and inhibit the LPS‐induced levels of NF‐κB activation and TNF‐ α.
APC can inhibit a number of pro‐inflammatory cytokines, chemokines and transcription factors in monocyte cell lines and normal human monocytes.30,31,32,33 Our results revealed two major EPCR‐dependent anti‐inflammatory effects of APC on peripheral blood monocytes from patients with RA. First, APC dose‐dependently inhibited the migration of RA monocytes toward MCP‐1, a chemoattractant that promotes the migration and activation of monocytes. Unlike granulocytes, when monocytes migrate into tissue, they survive for extended periods of time and can differentiate to become tissue‐resident macrophages. Mature macrophages can colonise the synovial sublining layer as well as the superficial and deep layers of the lining.34 Immunostaining showed that monocyte/macrophage‐like cells are a dominant cell population in the lining and sub‐lining layers of RA synovium (fig 1).
Second, APC markedly inhibited the activation of NF‐κB and the production of TNF‐α in RA monocytes via EPCR (figs 4 and 5). NF‐κB is a critical intracellular signaling pathway eliciting inflammatory mediators in monocytes/macrophages in RA synoviocytes.35 Inhibiting the activation of NF‐κB in macrophages is clinically beneficial in animal models of inflammatory arthritis36 and in patients with RA,37 as it prevents the inflammatory and destructive mechanisms but spares the anti‐inflammatory mediators. Similarly, TNF‐α is a critical inflammatory mediator involved in the pathogenesis of RA and is an important therapeutical target for RA.38,39,40
The fact that EPCR and APC18 are both anti‐inflammatory and elevated in RA appears to be paradoxical. However, the anti‐inflammatory effect exerted by APC/EPCR is unlikely to compensate for the disproportionate inflammatory activity in the RA joint. The elevated levels of EPCR/APC may represent a compensatory attempt to normalise monocyte activity. It is feasible that by increasing the levels of APC further, that is, by administering therapeutic doses, inflammation will be blocked.
In conclusion, this study has revealed that EPCR, the specific receptor for PC/APC, is expressed by RA synovium and is co‐localised with PC/APC on monocytes/macrophages. Furthermore, EPCR is required for APC to execute anti‐inflammatory effects on RA monocytes by inhibiting migration, NF‐κB activation and TNF‐α production. These multifactorial inhibitory effects on RA monocyte activation suggest that EPCR and APC may have novel therapeutic effects in RA.
Acknowledgements
This work was supported by a University of Sydney Postdoctoral Fellowship, Rebecca Cooper Foundation, Northern Sydney Area Health grant and the Sutton Arthritis Research Trust. We thank David Campbell for generous help, Susan Smith for preparation of synovial tissue sections, Ross Davey and Bill Walsh Cancer Research Laboratory for assistance in using fluorescent microscope.
Abbreviations
APC - activated protein C
EPCR - express endothelial protein C
IL - interleukin
LPS - lipopolysaccharide
MCP - monocyte chemoattractant protein
PC - protein C
RA - rheumatoid arthritis
TNF - tumour necrosis factor
Footnotes
Competing interests: None declared.
References
- 1.Firestein G S. Evolving concepts of rheumatoid arthritis. Nature 2003423356–361. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan F M, Maini R N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 199614397–440. [DOI] [PubMed] [Google Scholar]
- 3.Kinne R W, Brauer R, Stuhlmuller B, Palombo‐Kinne E, Burmester G R. Macrophages in rheumatoid arthritis. Arthritis Res 20002189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Athanasou N A. Current concepts review—cellular biology of bone‐resorbing cells. J Bone Joint Surg Am 1996781096–1112. [DOI] [PubMed] [Google Scholar]
- 5.Susa M, Luong‐Nguyen N H, Cappellen D, Zamurovic N, Gamse R. Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J Transl Med 200426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis [review]. Clin Exp Rheum 199311331–339. [PubMed] [Google Scholar]
- 7.Fukudome K, Esmon C T. Identification, cloning, and regulation of a novel endothelial cell protein c activated protein c receptor. J Biol Chem 199426926486–26491. [PubMed] [Google Scholar]
- 8.Xue M, Campbell D, Sambrook P N, Fukudome K, Jackson C J. Endothelial protein C receptor and protease‐activated receptor‐1 mediate induction of a wound‐healing phenotype in human keratinocytes by activated protein C. J Invest Dermatol 20051251279–1285. [DOI] [PubMed] [Google Scholar]
- 9.Galligan L, Livingstone W, Volkov Y, Hokamp K, Murphy C, Lawler M.et al Characterization of protein C receptor expression in monocytes. Br J Haematol 2001115408–414. [DOI] [PubMed] [Google Scholar]
- 10.Esmon C T. The anticoagulant and anti‐inflammatory roles of the protein C anticoagulant pathway. J Autoimmun 200015113–116. [DOI] [PubMed] [Google Scholar]
- 11.Esmon C T. Crosstalk between inflammation and thrombosis. Maturitas 200447305–314. [DOI] [PubMed] [Google Scholar]
- 12.Taylor F B J, Stearns‐Kurosawa D J, Kurosawa S, Ferrell G, Chang A C, Laszik Z.et al The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood 2000951680–1686. [PubMed] [Google Scholar]
- 13.Iwaki T, Cruz D T, Martin J A, Castellino F J. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide‐induced endotoxemia in the mouse. Blood 20051052364–2371. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Zheng X, Gu J, Hunter J, Ferrell G L, Lupu F.et al Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost 200531351–1359. [DOI] [PubMed] [Google Scholar]
- 15.Crawley J T, Gu J M, Ferrell G, Esmon C T. Distribution of endothelial cell protein C/activated protein C receptor (EPCR) during mouse embryo development. Thromb Haemost 200288259–266. [PubMed] [Google Scholar]
- 16.Gu J M, Crawley J T, Ferrell G, Zhang F, Li W, Esmon N L.et al Disruption of the endothelial cell protein C receptor gene in mice causes placental thrombosis and early embryonic lethality. J Biol Chem 200227743335–43343. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Zheng X, Gu J M, Ferrell G L, Brady M, Esmon N L.et al Extraembryonic expression of EPCR is essential for embryonic viability. Blood 20051062716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buisson‐Legendre N, Smith S, March L, Jackson C. Elevation of activated protein C in synovial joints in rheumatoid arthritis and its correlation with matrix metalloproteinase 2. Arthritis Rheum 2004502151–2156. [DOI] [PubMed] [Google Scholar]
- 19.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, C The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 20.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K.et al Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986291039–1049. [DOI] [PubMed] [Google Scholar]
- 21.Thakur A, Willcox M D. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res 200070255–259. [DOI] [PubMed] [Google Scholar]
- 22.Makarov S S. NF‐kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res 20013200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H.et al Involvement of interleukin‐8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha‐dependent angiogenesis. Mol Cell Biol 1997174015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calandra T, Bernhagen J, Mitchell R A, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 19941791895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M.et al An essential regulatory role for macrophage migration inhibitory factor in T‐celláactivation. PNAS 1996937849–7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oganesyan V, Oganesyan N, Terzyan S, Qu D, Dauter Z, Esmon N L.et al The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem 200227724851–24854. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Scherer D C, Singh N, Mendiratta S K, Serizawa I, Koezuka Y.et al Lipid antigen presentation in the immune system: lessons learned from CD1d knockout mice. Immunol Rev 199916931–44. [DOI] [PubMed] [Google Scholar]
- 28.Moody D B, Besra G S, Wilson I A, Porcelli S A. The molecular basis of CD1‐mediated presentation of lipid antigens. Immunol Rev 1999172285–296. [DOI] [PubMed] [Google Scholar]
- 29.Gong J H, Ratkay L G, Waterfield J D, Clark‐Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP‐1) inhibits arthritis in the MRL‐lpr mouse model. J Exp Med 1997186131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson D A, Toltl L J, Beaudin S, Liaw P C. Modulation of monocyte function by activated protein C, a natural anticoagulant. J Immunol 20061772115–2122. [DOI] [PubMed] [Google Scholar]
- 31.Brueckmann M, Hoffmann U, Dvortsak E, Lang S, Kaden J J, Borggrefe M.et al Drotrecogin alfa (activated) inhibits NF‐kappa B activation and MIP‐1‐alpha release from isolated mononuclear cells of patients with severe sepsis. Inflamm Res 200453528–533. [DOI] [PubMed] [Google Scholar]
- 32.Yuksel M, Okajima K, Uchiba M, Horiuchi S, Okabe H. Activated protein C inhibits lipopolysaccharide‐induced tumor necrosis factor‐alpha production by inhibiting activation of both nuclear factor‐kappa B and activator protein‐1 in human monocytes. Thromb Haemost 200288267–273. [PubMed] [Google Scholar]
- 33.Schmidt‐Supprian M, Murphy C, While B, Lawler M, Kapurniotu A, Voelter W.et al Activated protein C inhibits tumor necrosis factor and macrophage migration inhibitory factor production in monocytes. Eur Cytokine Netw 200011407–413. [PubMed] [Google Scholar]
- 34.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 199639115–124. [DOI] [PubMed] [Google Scholar]
- 35.Miyazawa K, Mori A, Yamamoto K, Okudaira H. Constitutive transcription of the human interleukin‐6 gene by rheumatoid synoviocytes: spontaneous activation of NF‐kappaB and CBF1. Am J Pathol 1998152793–803. [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell I K, Gerondakis S, O'Donnell K, Wicks I P. Distinct roles for the NF‐[kappa]B1 (p50) and c‐Rel transcription factors in inflammatory arthritis. J Clin Invest 20001051799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondeson J. The mechanisms of action of disease‐modifying antirheumatic drugs: A review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen Pharmacol Vasc Syst 199729127–150. [DOI] [PubMed] [Google Scholar]
- 38.Feldmann M, Maini R N. Anti‐TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 200119163–196. [DOI] [PubMed] [Google Scholar]
- 39.Leech M, Lacey D, Xue J R, Santos L, Hutchinson P, Wolvetang E.et al Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum 2003481881–1889. [DOI] [PubMed] [Google Scholar]
- 40.Mikulowska A, Metz C N, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II‐induced arthritis in mice. J Immunol 19971585514–5517. [PubMed] [Google Scholar]