Abstract
Background
Chimerism indicates the presence of cells from one individual in another individual, and has been associated with several autoimmune diseases. Although this finding may point towards a role for chimerism in the induction of SLE, it could also indicate that chimerism is the result of repair mechanisms after injury.
Objective
To perform a post‐mortem investigation for the presence of chimerism in 48 organs from seven women with SLE and establish whether there was a relationship between chimerism and injury.
Methods
Chimeric male cells in female tissue specimens were identified by in situ hybridisation of the Y‐chromosome. Organs were categorised into four different groups according to injury experienced. Results were compared with those for unaffected control organs.
Results
Chimerism was found in all seven patients with SLE. Y‐chromosome‐positive cells were present in 24 of 48 organs from women with SLE, which was significantly more than in control organs (p<0.001). Chimerism occurred more often in organs from patients with SLE who had experienced injury than in normal control organs, irrespective of whether the injury experienced was SLE‐related, non‐SLE‐related or both.
Conclusions
This is the first report of the distribution of chimerism in a large number of organs from women with SLE. It shows that the occurrence of chimerism is related to injury. The data support the hypothesis that tissue chimerism is the result of a repair process.
Chimerism is the presence of cells from one individual in another individual. The involvement of chimerism in the pathogenesis of autoimmune diseases has been addressed in several studies.1,2,3 We recently investigated the occurrence of chimerism in kidneys of women with the autoimmune disease systemic lupus erythematosus (SLE) and found that chimerism occurs twice as often in lupus nephritis as in normal kidneys.4 SLE is an immune‐mediated disease that affects several organs and has a variety of clinical symptoms.5 This disease is characterised by the presence of autoantibodies, particularly autoantibodies against nuclear components.6 Despite extensive research, the aetiology of SLE is still unknown, but is probably multifactorial. Chimerism is a candidate factor that may be responsible for the development of SLE.
SLE occurs in women and men at a ratio of approximately 10:1. In women, the first symptoms most often occur during their fertile years.7 This is interesting in terms of whether chimerism plays a role in SLE, because pregnancy is thought to be the most likely source of chimeric cells. During pregnancy, fetal cells enter the maternal circulation, making the mother chimeric. These circulating fetal cells have been reported to be haematopoietic progenitor cells, trophoblast cells, nucleated erythrocytes and leucocytes.8,9,10,11 We have shown that, in kidneys with lupus nephritis, both CD3 and CD34 positive chimeric cells are present.4
The presence of chimeric cells in tissues affected by SLE may indicate the pathogenic potential of chimeric cells. For example, their presence could be interpreted as a graft‐versus‐host or a host‐versus‐graft reaction.12 In these scenarios, chimeric cells are involved in the initiation of disease. However, recent publications have stressed the importance of chimeric cells in repair, showing in experimental designs that fetal cells migrate to sites of injury in the mother.13
Organ injury in autoimmune diseases such as SLE can be extensive. If chimeric cells indeed have repair capabilities, the amount of tissue chimerism would be expected to be high in organs from women with SLE, especially in those that show histological signs of injury. Moreover, it is possible that specifically SLE‐related injury leads to the presence of chimerism, in contrast with other forms of injury. In an autopsy case study of one patient with SLE, Johnson et al14 showed that chimerism was present in the heart, lung, kidney, intestines and skin. However, Khosrotehrani et al15 found no chimerism in skin specimens from seven patients with mild SLE. Apart from the 57 kidney specimens with lupus nephritis that we previously studied, there have been no other data gathered on either the occurrence of chimerism in organs from women with SLE or the relationship between tissue chimerism and injury.
Therefore, we investigated the association of chimerism and injury in a large number of tissue samples from patients with SLE, categorising whether these tissues had an SLE‐related injury, a non‐SLE‐related injury, a combination of the two, or were histologically normal.
Patients and methods
Patients
Tissue samples of the kidney, liver, heart, lung, spleen, lymph node, thyroid and skin were obtained at autopsy from seven women diagnosed with SLE during their life time. Autopsies were performed at the Leiden University Medical Center (LUMC) between 1985 and 2001. Clinical histories of patients were retrieved, including data on serology, disease course, therapy and cause of death. A pathologist, unaware of the clinical history and pathological findings stated in the autopsy report, reviewed the specimens.
Information on patient parity was derived from medical records or, if records were incomplete, by contacting general practitioners. Data on whether patients had received blood transfusions were obtained from the Department of Immunohematology and Blood Transfusion of the LUMC. Because these data were only available from 1987 onwards, we were not able to retrieve the blood transfusion history of one patient (patient 1).
Clinical histories
Patient 1 developed butterfly exanthema, alopecia and photosensibility at the age of 16 when SLE was diagnosed. Within a year, she developed nephrotic syndrome due to lupus nephritis, pericardial obliteration, pleuritis and serositis. Anti‐dsDNA antibodies were present. She received immunosuppressive therapy, but the disease never became quiescent. Cause of death at the age of 17 was liver failure caused by lupus hepatitis and acute necrotising pancreatitis. She had no children. We were not able to retrieve her blood transfusion history.
Patient 2 was diagnosed as having SLE at the age of 27, presenting with pleuritis and arthritis. Anti‐dsDNA antibodies were positive. Initially, the disease became quiescent under immunosuppressive therapy, but 6 months later an exacerbation with pleuritis and arthralgia developed. Immunosuppressive therapy was intensified, complicated by a cytomegalovirus infection. She was admitted to hospital, and in the course of 3 months, multiple manifestations of SLE became apparent (pleuritis, pericarditis and cerebral vasculitis). Together with multiple infections, these conditions led to her death at the age of 28. She had no children. She did not receive any blood transfusions.
Patient 3 developed tendinitis, arthritis, pleuritis, pericarditis and butterfly exanthema at the age of 26 when SLE was diagnosed. Antinuclear factor (ANF) was positive. She was treated with corticosteroids, and the disease became quiescent. At the age of 48 she had symptoms of polyneuropathy and, 2 years later, an exacerbation occurred with pericarditis. At the age of 53 she had surgery for an adenocarcinoma of the rectum. Eight years later, she presented with alopecia, oral ulcers, arthralgia and fever. Immunosuppressive therapy was intensified, but her condition was complicated by miliary tuberculosis, resulting in her death at age 61. She had no children. Shortly before her death she received two erythrocyte transfusions.
Patient 4 had onset of Raynaud's disease, pericarditis and vasculitis at the age of 24, which was originally diagnosed as mixed connective tissue disease. She received immunosuppressive therapy, which was initially successful. At the age of 33 she was admitted to hospital because of transverse myelitis and pulmonary hypertension, and SLE was diagnosed. ANF was positive. One month later, she died from severe pulmonary hypertension. At autopsy, lupus nephritis class II was found. She had no children. There was no history of blood transfusion.
Patient 5 developed pleuritis, pericarditis, SLE‐related cerebral symptoms, arthritis and butterfly exanthema, and was diagnosed as having SLE at the age of 38. Anti‐dsDNA antibodies and ANF were positive. Immunosuppressive therapy was started. The disease remained relatively quiescent, except for a period of cerebral ataxia, possibly SLE‐related, 9 years later. At the age of 54 she presented with dyspnoea caused by pleuritis, and was admitted to hospital. She appeared to have an exacerbation of SLE characterised by systemic vasculitis. This led to the development of mesenterial thrombosis, which was the cause of death. She had one son and one daughter. During the last 4 days before her death, she received 26 erythrocyte transfusions.
Patient 6 was diagnosed as having SLE at the age of 31. She presented with arthritis, exanthema, serositis and nephrotic syndrome due to lupus nephritis. ANF was positive. She was treated with immunosuppressive therapy, which was initially successful. Five years later, she had an exacerbation with a recurrence of the nephrotic syndrome. After remission, the disease remained quiescent for 15 years. At the age of 51, she presented with “general malaise” and fever, indicative of SLE recurrence. She was admitted to hospital, and developed rapidly progressive respiratory failure. Blood cultures were positive for Klebsiella oxytoca and Escherichia coli. She died from septic shock. At autopsy, active lupus nephritis was found, indicative of an SLE exacerbation. She had two daughters. In the last 2 months before her death, she received 17 leucocyte‐depleted transfusions and one undepleted erythrocyte transfusion.
Patient 7 had onset of skin abnormalities and arthritis at the age of 24. At 29, she developed pleuritis and nephrotic syndrome, and SLE was diagnosed. ANF was positive. Immunosuppressive therapy was initiated, and the disease became quiescent. Ten years later she developed a non‐Hodgkin lymphoma, for which she was treated with chemotherapy, resulting in partial remission of the lymphoma. However, 1 month later she developed a nephrotic syndrome as a result of recurrence of lupus nephritis. She died at the age of 39 from progressive congestive heart disease due to lupus myocarditis. At autopsy, no remnants of the non‐Hodgkin lymphoma were found. She had two children. She received eight packages of leucocyte‐depleted erythrocyte transfusions 1 week before her death.
Controls
Organs from patients with SLE were compared with 146 histologically normal organs from 34 control women. Control women were matched for age with the patients. Some of these data have been published.16 Organs were obtained at autopsy, performed at the LUMC between 1999 and 2001. Women with autoimmune disease were excluded. Of the 34 controls, eight had died from a vascular or myocardial cause, three from a cerebral cause, eight from an infectious cause, 13 from a malignancy, and two from other causes, namely amniotic fluid embolus and liver cirrhosis. Before being entered into the study, control organs were reviewed by light microscopy, and those that contained any histomorphological abnormalities, such as inflammatory infiltrates, scarring, atypia, hyperplasia or any other histologically apparent lesion, were excluded. The remaining 146 control specimens were histomorphologically normal. Blood transfusion status and patient parity were retrieved by the methods used for the patients with SLE.
In situ hybridisation
To detect chimeric cells, in situ hybridisation of the Y‐chromosome was performed as described previously.4 Male tissue samples served as positive controls; they contained red–brown dots confirming a positive signal in 70% of the nuclei. We confirmed by PCR and sequencing that the probe was specific for the Y‐chromosome.16. As a negative control, a male tissue sample was used on which the complete in situ hybridisation protocol was performed, but instead of hybridisation mixture with the Y‐chromosome probe, only hybridisation mixture was added. This negative control was consistently negative.
Scoring
All organ specimens of patients were scored for the presence of Y‐chromosome‐positive cells by light microscopy. Two observers, blinded to the clinical and histological information of the patients, scored the specimens. For all specimens, a random area of 58 mm2 was scored. A template was used to draw this area on the cover slide, to ensure that both observers scored the same area. From clinical data and histology, it appeared that organs derived from women with SLE had different injury histories, which could be divided into four groups: (1) injury that could be related to SLE; (2) injury not related to SLE; (3) injury that was both SLE‐related and non‐SLE‐related; (4) no history of injury and no histological abnormalities. SLE‐related injury included pleuritis, pericarditis, lupus nephritis, periarterial fibrosis of the spleen and lupus hepatitis. In the case of skin specimens, five patients had experienced butterfly exanthema of the facial skin, but facial skin was never included in the tissue block at autopsy. From these patients, skin specimens of other areas that were normal were put in group 4, the normal tissue group. If an organ had experienced various types of injury, but the dominant lesion was SLE‐related, the organ was categorised as group 1 (SLE‐related injury). This was the case for five organs. Five organs had equally prominent SLE‐related and non‐SLE‐related injury, and were included in the third group.
Tissue slides were also scored for the presence of inflammatory infiltrates according to the following criteria: 0 = no foci of inflammatory cells; 1 = minor infiltrates, mostly in a diffuse pattern throughout the tissue specimen; 2 = more diffuse infiltrates and foci with a vast amount of inflammatory cells (diffuse/focal pattern); 3 = highly diffuse infiltrates throughout the tissue specimen with focal areas of abundant inflammatory cells. Kidney specimens with lupus nephritis were also scored according to the most recent modification of the WHO classification.17
Statistical analysis
Categorical variables were compared by χ2 test. Continuous variables were compared by Student t test. Correlations were determined by calculating point‐biserial correlations.
Results
Seven women with SLE were included in the study (age range 17–61 years; mean age 41) and 34 control women (age range 16–61; mean age 47). In the patients with SLE, the time since diagnosis ranged from 1 month to 20 years (mean 7 years). Age at diagnosis ranged from 16 to 38 years.
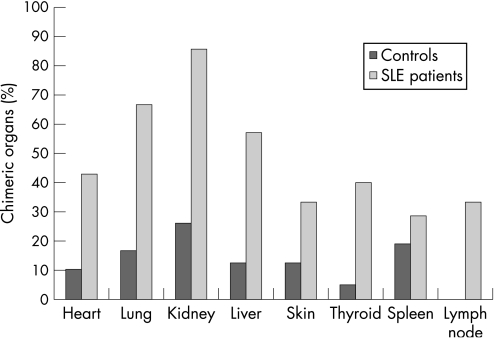
Chimeric cells were found in all seven patients with SLE, whereas chimerism only occurred in 15 of the 34 (44%) control women. Comparing the results for the individual organs, we found that Y‐chromosome‐positive chimeric cells were present in 24 of 48 (50%) organs derived from women with SLE, and in 21 of 146 (14%) organs from controls (p<0.001). Table 1 shows that chimeric cells were present at least once in every kind of organ, both in patients with SLE and controls, except for lymph nodes, which were negative in the control group. There was no significant difference in the occurrence of chimerism between the different organs within the patient or control group (fig 1).
Table 1 Occurrence of chimerism in organs from patients with systemic lupus erythematosus (SLE) and controls.
| Patients with SLE (n = 7) | Controls (n = 34) | |||
|---|---|---|---|---|
| Chimerism present (%) | Chimerism absent (%) | Chimerism present (%) | Chimerism absent (%) | |
| Heart | 3 (43) | 4 (57) | 3 (10) | 26 (90) |
| Lung | 4 (67) | 2 (33) | 3 (17) | 15 (83) |
| Kidney | 6 (86) | 1 (14) | 6 (26) | 17 (74) |
| Liver | 4 (57) | 3 (43) | 3 (13) | 21 (87) |
| Spleen | 2 (33) | 4 (67) | 4 (19) | 17 (81) |
| Skin | 2 (33) | 4 (67) | 1 (13) | 7 (87) |
| Thyroid | 2 (40) | 3 (60) | 1 (5) | 19 (95) |
| Lymph node | 1 (33) | 2 (67) | 0 (0) | 3 (100) |
Figure 1 Occurrence of chimerism in organs of women with systemic lupus erythematosus (SLE) and control women.
Table 2 lists specified data on occurrence and number of chimeric cells in organs from women with SLE. The number of chimeric cells ranged from one to five per scoring area of 58 mm2 in all instances. Groups of Y‐chromosome‐positive cells were not found, except for a cluster of five chimeric cells in the lung of patient 5.
Table 2 Characteristics of seven women with systemic lupus erythematosus and the number of male cells found in their organ specimens.
| Children | Blood transfusion | Anti‐ sDNA antibodies | Immunosuppressive therapy | Lupus nephritis class | Heart | Lung | Kidney | Liver | Spleen | Skin | Thyroid | Lymph node | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 0 | NA | +ve | Yes, but specific drugs unknown | II + V | 0 | 2 | 5 | 2 | 0 | 0 | 1 | NA |
| Patient 2 | 0 | No | +ve | PR, HC, CY (1 shot) | No lupus nephritis | 3 | 2 | 1 | 0 | 0 | NA | NA | 1 |
| Patient 3 | 0 | Yes | −ve | PR and HC | III (A/C) | 2 | NA | 2 | 0 | 0 | 1 | 3 | NA |
| Patient 4 | 0 | No | −ve | PR and AZ | II | 0 | 0 | 2 | 1 | 0 | 1 | NA | NA |
| Patient 5 | 2 | Yes | +ve | PR and AZ | II | 3 | 5 | 0 | 0 | 2 | 0 | 0 | 0 |
| Patient 6 | 2 | Yes | −ve | PR, AZ, CY (1 shot) and IG (2 days) | III (A) | 0 | 1 | 1 | 2 | 0 | 0 | 0 | NA |
| Patient 7 | 2 | Yes | NA | PR and CY | V | 0 | 0 | 5 | 1 | 1 | 0 | 0 | 0 |
NA, not applicable.
Immunosuppressive therapy ever had: PR, prednisone; HC, hydroxychloroquine; CY, cyclophosphamide; AZ, azathioprine; IG, immunoglobulins.
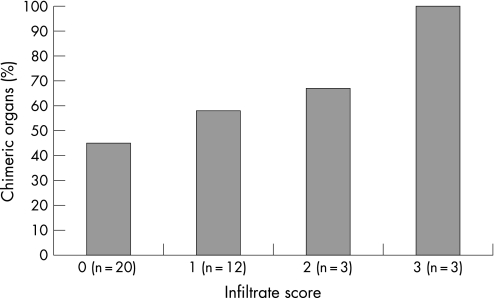
All organs were evaluated by a pathologist for the presence of cellular infiltrates. Although increased occurrence of chimerism seemed to correspond to higher infiltrate scores, this relationship was not significant (fig 2; p = 0.32, correlation coefficient = 0.3). Only 20% of chimeric cells were found inside or near an inflammatory infiltrate.
Figure 2 Occurrence of chimerism related to the infiltrate score of organs from women with systemic lupus erythematosus. Tissue slides were scored for the presence of inflammatory infiltrates according to the following criteria: 0 = no foci with inflammatory cells; 1 = minor infiltrates, mostly in a diffuse pattern throughout the tissue specimen; 2 = more diffuse infiltrates and foci with a vast amount of inflammatory cells (diffuse/focal pattern); 3 = highly diffuse infiltrates throughout the tissue specimen with focal areas of abundant inflammatory cells.
Three women with SLE had children and four had no children. Four patients with SLE had received a blood transfusion, two had not, and for one patient we could not retrieve her blood transfusion status. Table 2 shows data on children and blood transfusion history. Although two women had not received a blood transfusion and had no children, chimerism was found in all seven patients with SLE. In the control group, 22 women had children, and 12 had no children. Of the 15 control women whose organs exhibited chimerism, 11 had had a blood transfusion. Similarly, of the 19 non‐chimeric control women, 11 had received a blood transfusion. In neither the patient group nor the controls did we find a relationship between the presence of chimeric cells and either patient parity or history of blood transfusion.
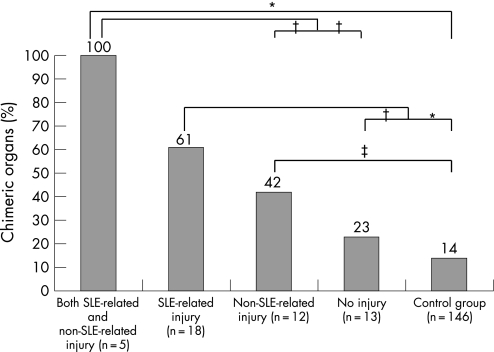
Of the organs from patients with SLE, five had experienced both SLE‐related injury and non‐SLE‐related injury, 18 had experienced only SLE‐related injury, 12 had experienced non‐SLE‐related injury, and 13 had no abnormalities. Figure 3 shows the distribution of chimeric cells within the four categories of organ injury, both for patients with SLE and controls. Chimerism occurred significantly more often in organs derived from women with SLE that showed SLE‐related injury compared with organs from women with SLE without injury (p = 0.036) and control organs (p<0.001). Chimerism was also found significantly more often in organs that had experienced injury that was not SLE‐related compared with controls (p = 0.014). Chimerism was found in all five organs with both SLE‐related and non‐SLE‐related injury.
Figure 3 Occurrence of chimerism related to organ injury in women with systemic lupus erythematosus (SLE). According to the criteria described in the text, organs from women with SLE were categorised into four groups on the basis of injury experience. The percentage of chimeric organs in these four groups and in normal control organs is given. *p⩽0.001; †p<0.05; ‡p<0.015.
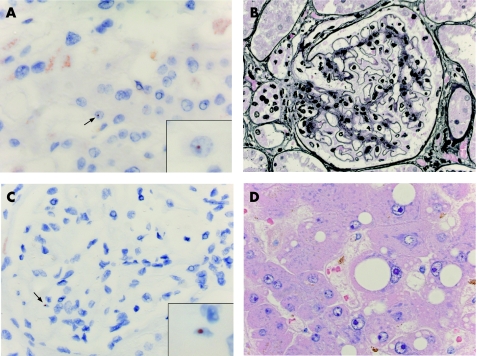
We observed that the level of chimerism corresponded to the degree of organ injury. We found the least amount of chimerism in normal organs from healthy controls, followed by normal organs from patients with SLE, organs of patients with non‐SLE‐related injury, organs of patients with SLE‐related injury, and the most chimerism was seen in organs affected by both SLE and another type of injury. Chimerism was found significantly more often in organs from patients with SLE that experienced injury compared with normal control organs, irrespective of whether the injury was SLE‐related, non‐SLE‐related or both. Figure 4 shows two examples of chimerism in organs from women with SLE with different kinds of injury.
Figure 4 (A) Extensive steatosis in a liver specimen from a woman with systemic lupus erythematosus (SLE) (H&E staining). (B) Y‐chromosome‐positive cell (arrow, detail in inset) in the same liver as in (A). Red–brown dot indicates the Y‐chromosome, as identified by in situ hybridisation. (C) Lupus nephritis WHO class II in a renal specimen from a woman with SLE (silver staining). (D) Y‐chromosome‐positive cell (arrow, detail in inset) in the same kidney as in (C).
Discussion
We investigated the distribution of Y‐chromosome‐positive chimeric cells in 48 organs from seven female patients with SLE. Y‐chromosome‐positive chimeric cells were identified significantly more often in organs from women with SLE than in control organs. Overall, our results show that there is a relationship between the presence of chimerism and organ injury. In patients with SLE, there was no significant difference in the occurrence of chimerism between organs with SLE‐related injury and those with a non‐SLE‐related injury. This may indicate that chimerism is involved in various types of injury. The occurrence of chimerism was the same in normal organs from patients with SLE and normal organs from healthy controls. This demonstrates that patients with SLE do not have a higher “background level” of chimeric cells than normal controls.
Our finding that chimerism is related to injury experienced in organs from women with SLE could be interpreted in several ways. One possibility is that chimeric cells are involved in repair mechanisms, a hypothesis put forward previously known as the “repair hypothesis”.12,18,19 The repair hypothesis suggests that chimeric cells develop from progenitor cells into parenchymal cells and replace damaged host cells after tissue injury. This idea came from studies that reported that Y‐chromosome‐positive cells with a particular phenotype (eg, cytokeratin or Heppar‐1‐positive) were present in human tissues affected by various diseases.20 In an experimental mouse model, Wang et al13 showed that, in gentamicin‐induced kidney injury and ethanol‐induced liver injury, fetus‐derived chimeric cells migrated to the injured tissues. Recently, Khosrotehrani et al21 found fetus‐derived chimeric cells in chemically injured livers and spleens of mice.
A relationship between chimerism and injury has not been previously reported in human studies on SLE. In fact, there are data that contradict this hypothesis. Khosrotehrani et al15 found no chimeric cells in seven skin samples of women affected with mild SLE. They suggested that, in SLE, chimeric cells probably only migrate to affected tissues if the damage reaches a particular threshold. The only study so far to investigate chimerism in various organs in a patient with SLE was a case report by Johnson et al,14 reporting chimerism in heart, lung, kidney, intestines and skin. Interestingly, the highest number of chimeric cells was found in the intestines, in which extensive damage caused by infection and drugs was present. They concluded that chimeric cells were associated with pathological processes involved in SLE. We are the first to investigate thoroughly the relationship between chimerism in organs from patients with SLE, making a distinction between SLE‐related and non‐SLE‐related injury. Our results are in line with the repair hypothesis, demonstrating that, in comparison with organs from patients with SLE that were normal, both those with SLE‐related and non‐SLE‐related injury showed a high occurrence of Y‐chromosome‐positive chimeric cells. However, this theory remains as yet unproven in humans. Final proof requires a dedicated study design.
Another possibility is that organs in which injury is taking place produce more cytokines, leading to the recruitment of more inflammatory cells, including chimeric cells. To test this possibility, we scored for infiltrates and examined for every chimeric cell found whether it was located inside an inflammatory infiltrate or whether an inflammatory infiltrate was present in the same microscopic visual field as the chimeric cell. Our data show that indeed there was a tendency towards an increased occurrence of chimerism whenever inflammatory infiltrates were present. However, careful observation of the location of the chimeric cells showed that chimeric cells were only present inside an infiltrate in five tissue specimens. Therefore, the high occurrence of chimeric cells in patients with SLE cannot be explained solely by an overall increase in cells due to the influx of inflammatory cells as a result of response to injury.
As all the patients had received immunosuppressive therapy during their lifetimes, a third possibility is that they were not able to build up an effective immune response to clear chimeric cells. On the other hand, controls, with an intact immune system, may exhibit chimeric cells in their organs less often. However, this theory does not explain why chimeric cells are especially present in organs that have experienced injury.
Although it is assumed that the main source of chimerism is pregnancy, we found Y‐chromosome‐positive cells in patients both with and without children. This phenomenon has been described in other studies investigating chimerism in autoimmune diseases.16,22,23 The presence of chimerism in patients without children can be explained by miscarriages, unrecognised pregnancies, and/or blood transfusions.24,25,26 In the present study, we were not able to retrieve information on the first two sources. Data on blood transfusions were available. However, two women with SLE were found to be chimeric without having had children or blood transfusions.
This study describes for the first time the distribution of chimeric cells in a large number of organs derived from women with SLE, and shows that all seven women with SLE were chimeric. Furthermore, a relationship between the presence of chimerism and organ injury is shown. Our findings suggest that the repair capacities of chimeric cells may be involved. However, we cannot exclude the possibility that chimeric cells are immunological cells that, under certain circumstances, may become targets of a detrimental immune response leading to the development of SLE.
Acknowledgements
We thank the Gratama Foundation for their support.
Abbreviations
ANF - antinuclear factor
SLE - systemic lupus erythematosus
Footnotes
Funding: MK and IKH received financial support from the Gratama Foundation, a non‐commercial foundation. The Gratama Foundation did not participate in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Competing interests: None.
References
- 1.Ando T, Davies T F. Self‐recognition and the role of fetal microchimerism. Best Pract Res Clin Endocrinol Metab 200418197–211. [DOI] [PubMed] [Google Scholar]
- 2.Nelson J L. Maternal‐fetal immunology and autoimmune disease: is some autoimmune disease auto‐alloimmune or allo‐autoimmune? Arthritis Rheum 199639191–194. [DOI] [PubMed] [Google Scholar]
- 3.Nelson J L. Pregnancy, persistent microchimerism, and autoimmune disease. J Am Med Womens Assoc 19985331–2, 47. [PubMed] [Google Scholar]
- 4.Kremer Hovinga I C, Koopmans M, Baelde H J, Wal A M, Sijpkens Y W, de Heer E.et al Chimerism occurs twice as often in lupus nephritis as in normal kidneys. Arthritis Rheum 2006542944–2950. [DOI] [PubMed] [Google Scholar]
- 5.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 6.Sherer Y, Gorstein A, Fritzler M J, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 200434501–537. [DOI] [PubMed] [Google Scholar]
- 7.Cervera R, Abarca‐Costalago M, Abramovicz D, Allegri F, Annunziata P, Aydintug A O.et al Systemic lupus erythematosus in Europe at the change of the millennium: lessons from the “Euro‐Lupus Project”. Autoimmun Rev 20065180–186. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi D W, Flint A F, Pizzimenti M F, Knoll J H, Latt S A. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc Natl Acad Sci USA 1990873279–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi D W, Zickwolf G K, Weil G J, Sylvester S, DeMaria M A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 199693705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans P C, Lambert N, Maloney S, Furst D E, Moore J M, Nelson J L. Long‐term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood 1999932033–2037. [PubMed] [Google Scholar]
- 11.Mueller U W, Hawes C S, Wright A E, Petropoulos A, DeBoni E, Firgaira F A.et al Isolation of fetal trophoblast cells from peripheral blood of pregnant women. Lancet 1990336197–200. [DOI] [PubMed] [Google Scholar]
- 12.Kremer Hovinga I C, Koopmans M, de Heer E, Bruijn J A, Bajema I M. Chimerism in systemic lupus erythematosus: three hypotheses. Rheumatology (Oxford) 200746200–208. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Iwatani H, Ito T, Horimoto N, Yamato M, Matsui I.et al Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Biophys Res Commun 2004325961–967. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K L, McAlindon T E, Mulcahy E, Bianchi D W. Microchimerism in a female patient with systemic lupus erythematosus. Arthritis Rheum 2001442107–2111. [DOI] [PubMed] [Google Scholar]
- 15.Khosrotehrani K, Mery L, Aractingi S, Bianchi D W, Johnson K L. Absence of fetal cell microchimerism in cutaneous lesions of lupus erythematosus. Ann Rheum Dis 200564159–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koopmans M, Kremer Hovinga I C, Baelde H J, Fernandes R J, de Heer E, Bruijn J A.et al Chimerism in kidneys, livers and hearts of normal women: implications for transplantation studies. Am J Transplant 200551495–1502. [DOI] [PubMed] [Google Scholar]
- 17.Weening J J, D'Agati V D, Schwartz M M, Seshan S V, Alpers C E, Appel G B.et al The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 200415241–250. [DOI] [PubMed] [Google Scholar]
- 18.Adams K M, Nelson J L. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA 20042911127–1131. [DOI] [PubMed] [Google Scholar]
- 19.Khosrotehrani K, Bianchi D W. Multi‐lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci 20051181559–1563. [DOI] [PubMed] [Google Scholar]
- 20.Khosrotehrani K, Johnson K L, Cha D H, Salomon R N, Bianchi D W. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 200429275–80. [DOI] [PubMed] [Google Scholar]
- 21.Khosrotehrani K, Reyes R R, Johnson K L, Freeman R B, Salomon R N, Peter I.et al Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod 200722654–661. [DOI] [PubMed] [Google Scholar]
- 22.Lambert N C, Pang J M, Yan Z, Erickson T D, Stevens A M, Furst D E.et al Male microchimerism in women with systemic sclerosis and healthy women who have never given birth to a son. Ann Rheum Dis 200564845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Z, Lambert N C, Guthrie K A, Porter A J, Loubiere L S, Madeleine M M.et al Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med 2005118899–906. [DOI] [PubMed] [Google Scholar]
- 24.Khosrotehrani K, Johnson K L, Lau J, Dupuy A, Cha D H, Bianchi D W. The influence of fetal loss on the presence of fetal cell microchimerism: a systematic review. Arthritis Rheum 2003483237–3241. [DOI] [PubMed] [Google Scholar]
- 25.Lambert N C, Lo Y M, Erickson T D, Tylee T S, Guthrie K A, Furst D E.et al Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood 20021002845–2851. [DOI] [PubMed] [Google Scholar]
- 26.Lee T H, Paglieroni T, Ohto H, Holland P V, Busch M P. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long‐term microchimerism in severe trauma patients. Blood 1999933127–3139. [PubMed] [Google Scholar]