Abstract
We cloned the mouse homologue of the chemokine receptor CXCR3, which is located in mouse chromosome X. We screened a large panel of chemokines for their ability to induce a calcium flux in mouse CXCR3-transfected cells and identified a new ligand for this receptor, the recently reported CC chemokine 6Ckine. This represents an example of a CC chemokine, which binds to a CXC chemokine receptor. Like other ligands of this receptor, 6Ckine has angiostatic properties. 6Ckine is known to chemoattract T cells. In line with this, CXCR3 is expressed preferentially in Th1 cells and in lymphoid organs of the IL-10−/− mouse that develops chronic colitis. Its ability to attract T cells as well as its angiostatic properties suggest that 6Ckine may be an effective anti-tumor agent.
Chemokines are a family of molecules composed of at least 50 different leukocyte chemotractants, which are produced during inflammation and regulate leukocyte migration (1). These proteins are 68–120 amino acids in length and have been divided into four structural categories (2) based on the number and arrangement of their conserved cysteines: the CXC, CC, and CX3C groups have four conserved cysteines, whereas the C chemokine (lymphotactin) has only two (3). A membrane bound chemokine with a CX3C motif also has been described (4). Most CXC chemokines are chemotactic for neutrophils, whereas the CC generally attract monocytes and lymphocytes; the C chemokine is specific for T and NK lymphocytes (5), and the CX3C, like the CC chemokines, appears to be a potent chemoattractant for monocytes and lymphocytes but not for neutrophils. Leukocytes respond to chemokines through G protein-coupled receptors (GPCR). These receptors belong to a large and functionally diverse superfamily of proteins containing seven transmembrane domains (7TMD). A common feature of signaling through the known chemokine receptors is that their signal transduction pathway results in the release of intracellular Ca2+ and is sensitive to pertussis toxin (6), indicating that most chemokine receptors are linked to the Gi class proteins. Chemokine receptors have been divided into five separate classes: (i) CXC restricted, (ii) CC restricted, (iii) CC and CXC restricted, (iv) orphans (with ligands undefined), and (v) viral. In humans, five CXC chemokine receptors (CXCR1–5) and nine CC chemokine receptors (CCR1–9) have been described so far based on their ligand specificity: CXCR1 for IL-8, GRO-α, NAP-2 (binding with different affinities), and GCP-2 (7); CXCR2 for IL-8, GRO-α, NAP-2 (binding with the same affinity), and GCP-2 (8); CXCR3 for IP-10 and MIG (9); CXCR4 for SDF-1 (10), and CXCR5 (BRL-1) for a new CXC chemokine (11).
Here, we describe the cloning and characterization of mouse CXCR3 (mCXCR3). We identified a new ligand for this receptor, the recently described CC chemokine 6Ckine (5) [also called exodus 2 (12) or SLC (13)], which will hereafter be referred to as 6Ckine. This constitutes an example of a CC chemokine that specifically binds a CXC receptor.
MATERIALS AND METHODS
Cloning of mCXCR3.
Part of the mouse CXCR3 chemokine receptor was amplified by PCR from a TCRαβ+ CD4−CD8− thymocyte cDNA library, prepared in pJFE-14. Two degenerate oligonucleotide primers corresponding to a conserved motif of chemokine receptors (known as the DRY box) [GA(T/C)(A/C)GNTA(T/C)CT(A/G)GC(N)TA(CAG)GT(N) CA(T/C)GC] and to the TMD-7 [GGGTCATGA IGGGTTIAI(G/A)CA(G/A)C(T/A)(G/A)(T/C)G] were used in the PCR reactions. Fifty microliter reaction mixtures containing 1 μg of cDNA, 1× GeneAmp buffer (Perkin–Elmer), 1.5 mM MgCl2, 250 μM each deoxynucleotide, 1 μM primers, and 2.5 units of AmpliTac DNA polymerase (Perkin–Elmer) were subjected to 30 cycles (94°C for 1 min, 55°C for 1 min, and 72°C for 2 min) on a Thermal Cycler (Perkin–Elmer). All of the PCR products were cloned into the pCR 2.1 vector (Invitrogen) and sequenced. A PCR product of 237 nucleotides showed 80% nucleotide sequence identity with human CXCR3. The 237-bp probe was used for screening of the αβ+TCRCD4−CD8− T-cell cDNA library. Four full length clones were isolated and sequenced to completion.
Tissue Distribution of mCXCR3.
The tissue distribution of mCXCR3 was analyzed by Northern analysis of multiple tissue Northern blots (CLONTECH) with 32P-labeled 237-bp PCR product and with the full length clone, both of which were labeled by random priming using a Prime-It kit (Stratagene). The distribution of CXCR3 also was analyzed in cDNA libraries by Southern blotting as described (14).
Generation of mCXCR3-Transfected Cells.
The mCXCR3 cDNA was amplified using primers containing 5′-ClaI and 3′-NotI restriction enzyme sequences. The PCR fragment was ligated into the same sites of an expression vector pFLAG–CMV3 (Kodak) containing a N-terminal FLAG fusion sequence. The pFLAG–CMV3–mCXCR3 construct was used to transfect HEK293 cells by LipofectAmine (GIBCO/BRL). Stable transfectants were generated by selection with 400 μg/ml G418 for 2 wk. The mCXCR3-expressing cells were further selected by a fluorescence-activated cell sorter by using biotin-conjugated M2 anti-FLAG antibody (Eastman Kodak) to obtain a population in which >95% of the cells expressed the receptor.
Calcium Flux Assay.
HEK293 cells stably expressing mCXCR3 were suspended in 1 × 107/ml in DMEM with 10% fetal calf serum. The cells were loaded with 3 μM Indo-1 acetoxymethyl ester (Molecular Probes) at room temperature in the dark for 45 min. The loaded cells were washed and resuspended in Hank’s balanced salt buffer (2 mM CaCl2/145 mM NaCl/5 mM KCl/1 mM MgCl2/5 mM d-glucose/20 mM Hepes, pH 7.3) with 1% fetal calf serum at the same concentration. Calcium mobilization of 1 × 106 cells was measured in 2 ml of Hanks’ balanced salt solution with 1.6 mM CaCl2 in a continuously stirring acrylic cuvette at 37°C in a Photon Technologies spectrofluorimeter (Princeton). The fluorescence was monitored as ratio of emission at 405 and 483 nm at an excitation wavelength of 350 nm. All the chemokines listed in Table 1 were tested in concentrations ranging from 100 nM to 1 μM.
Table 1.
Chemokines tested in calcium flux experiment for CXCR3 transfectants
| Chemokine | Calcium flux |
|---|---|
| IP-10 | + |
| Mig | + |
| 6Ckine | + |
| IL-8 | − |
| Gro α | − |
| Nap-2 | − |
| H-CC4 | − |
| Mip-3α | − |
| Mip-3β | − |
| TECK | − |
| MDC | − |
| TARC | − |
| Eotaxin 2 | − |
| DC-CK-1 | − |
| hI-309 | − |
Corneal Micropocket Model of Angiogenesis.
In vivo angiogenic activity was assayed in the avascular cornea of Long Evans rat eyes, as described (15). In brief, cytokines were combined with sterile Hydron (Interferon Sciences, New Brunswick, NJ) casting solution, and 5 μl of aliquots were air-dried on the surface of polypropylene tubes. Before implantation, pellets were rehydrated with normal saline. Animals were anesthetized with an i.p. injection of ketamine (150 mg/kg) and atropine (250 μg/kg). Rat corneas were anesthetized with 0.5% proparacaine hydrochloride ophthalmic solution followed by implantation of the Hydron pellet into an intracorneal pocket (1–2 mm from the limbus). Six days after implantation, animals were pretreated i.p. with 1,000 units of heparin (Elkins-Sinn, Cherry Hill, NJ), anesthetized with ketamine (150 mg/kg), and perfused with 10 ml of colloidal carbon via the left ventricle. Corneas were then harvested and photographed. No inflammatory response was observed in any of the corneas treated with the above reagents. Positive neovascularization responses were recorded only if sustained directional ingrowth of capillary sprouts and hairpin loops toward the implant were observed. Negative responses were recorded when either no growth was observed or when only an occasional sprout or hairpin loop displaying no evidence of sustained growth was detected. Positive controls included basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). Angiostatic activity was demonstrated as the ability of a molecule to inhibit the angiogenic activity of bFGF or VEGF.
Interspecific Mouse Backcross Mapping.
Interspecific backcross progeny were generated by mating (C57BL/6J × Mus spretus)F1 females and C57BL/6J males as described (16). A total of 205 N2 mice were used to map the Cxcr3 locus. Southern blot transfer and hybridization were performed as described (17). The probe, an ≈1.1-kb NotI/BamHI fragment of mCXCR3, was labeled with [α-32P]dCTP and washing to a final stringency of 1× standard saline citrate phosphate (SSCP; 1× SSCP = 120 mM NaCl/5 mM sodium citrate/20 mM sodium phosphate, pH 6.8 ) and 0.1% SDS, 65°C. A fragment of 14.0 kb was detected in EcoRI-digested M. spretus DNA. The presence or absence of the 7.8-kb EcoRI M. spretus-specific fragment was followed in backcross mice.
A description of the probes and restriction fragment length polymorphisms for the loci linked to Cxcr3 including Efnb1 and Pou3f4 has been reported previously (17). Recombination distances were calculated by using map manager, version 2.6.5 (17). Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns.
Chromosomal Mapping of Human CXCR3.
150 ng of Genomic DNA from a panel of 20 human–rodent somatic hybrid cell lines (Bios, New Haven, CT) were used as a template in a 50 μl of PCR reaction. The human CXCR3 primer pairs were: 5′ sense primer ATGGTCCTTGAGGTGAGTGACCAC and 3′ antisense primer TCACAAGCCCGAGTAGGAGGCCTC. Total human genomic DNA was used as positive control. Chinese hamster and mouse genomic DNA were used as negative controls.
RESULTS
Cloning of mCXCR3.
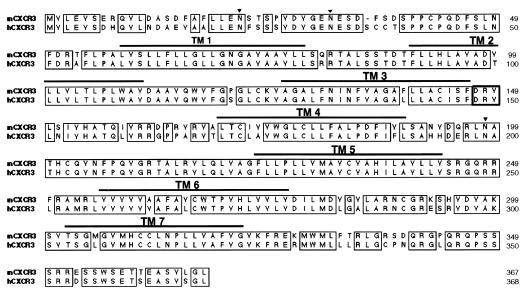
Chemokine receptors belong to the GPCR family and exhibit common structural features. We initially sought to identify new chemokine receptors expressed by αβTCRCD4−CD8−T cells. To this end, we designed degenerate primers targeting the conserved DRY box and seventh transmembrane (TM7) consensus sequences and amplified a 237-bp product from αβTCR+CD4−CD8− cDNA library. Sequencing of this PCR product indicated that it shared significant homology with GPCRs, but its sequence did not correspond to known receptors. This PCR product was used as a probe for screening of the cDNA library from αβTCR+CD4−CD8− T cells. A 1,626-bp clone encoding the full-length receptor was identified subsequently. The full sequence of this clone indicated a high degree of nucleotide sequence homology to human CXCR3 (hCXCR3), and its amino acid sequence was nearly identical (Fig. 1). This cDNA has an ORF of 1,101 bp and encoded a 367 amino acid protein with a predicted molecular weight of 41,014. The predicted amino acid sequence exhibits the four highly conserved cysteine residues in the extracellular region, three potential N-glycosylation sites: Asn22, Asn32, and Asn198 (also present in the human version), and three threonine and eight serine residues that are present in the intracellular COOH-terminal region, as potential phosphorylation sites which are common among this family of receptors. The receptor also has the HCCXNP motif in the seventh TMD.
Figure 1.
Aligment of the mCXCR3 predicted amino acid sequence with hCXCR3. The arrowhead indicates potential N-linked glycosilation sites and the horizontal lines the putative TMD (TMD1–TMD7). The alignment was generated by using clustalw (17). The sequence data are available from European Molecular Biology Laboratory (EMBL)/GenBank under accession no. AF045146.
Expression Pattern.
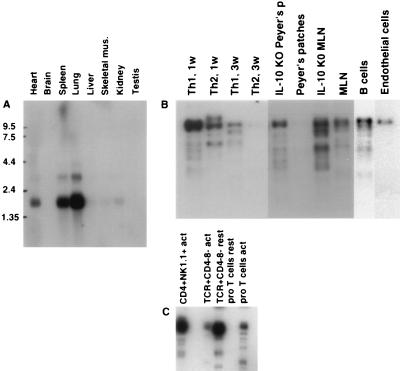
Northern blot analysis showed a 1.7-kb mRNA present mainly in lung, spleen, and heart and a very low but detectable amount in testis, kidney, skeletal muscle, and liver. A larger, 3.6-kb mRNA was detected in lung, spleen, and heart (Fig. 2A), suggesting that CXCR3 expression may not be T cell specific. Southern blot analysis of various cDNA libraries (Fig. 2B) indicated that CXCR3 mRNA is preferentially transcribed in CD4+ Th1-polarized T cells and that this difference (between Th1- and Th2-polarized populations) increases with time. CXCR3 message also was detected in CD4+NK1.1+ and αβTCR+CD4−CD8− thymocytes (Fig. 2C) and in CD8+ T cells (data not shown). Interestingly, CXCR3 was more abundant in a cDNA library from resting αβTCR+CD4−CD8− than in a corresponding cDNA library from CD3-activated αβTCR+CD4−CD8− (Fig. 2C). The latter phenomenon (down-regulation upon activation) also was observed in a Th1 cell clone (D1.1) after activation with Con A (data not shown). Conversely, CXCR3 is up-regulated upon activation in pro-T thymocytes (Fig. 2C). This indicates that CXCR3 is differentially regulated in various T cell subsets. Given the correlation of CXCR3 expression with the Th1 CD4 subset (18), we then investigated its expression in animal models in which Th1 cells are believed to play a role. As shown in Fig. 2B, CXCR3 is up-regulated in cDNA libraries from mesenteric lymph nodes and Peyer’s patches from the IL-10−/− mouse, suggesting the presence of Th1 cells in these organs. In fact, polarization of the Th cell subsets is likely to occur mainly in the secondary lymphoid organs (18).
Figure 2.
Distribution of mRNA of mCXCR3. (A) Multiple tissue Northern blot was hybridized with a full-length mCXCR3 cDNA probe, and two bands of 1.7 and 3.6 kb were detected in lung, spleen, and heart. (B) Southern blot analysis of different cDNA libraries: Th1 1 wk, naive cells from spleen were polarized for 7 days with IFNγ and anti-IL-4; Th2 1 wk, naive cells were polarized for 7 days with IL-4 and anti-IFNγ. Th1 3 wk: naive cells from Rag1−/− × DO-11.10-transgenic mice were stimulated with ovalbumin-(323–339) peptide in the presence of irradiated splenic APC and IL-12 and anti-IL-4 for 3 wk. Th2 3 wk: naive cells from transgenic mice (similar to Th1 3 wk) were stimulated with anti-IL-12 plus IL-4 for 3 wk; IL-10−/− mice Peyer’s patches; Peyer’s patches; IL-10−/− mice mesenteric lymph nodes; mouse mesenteric lymph nodes; B cells derived from spleen; and mouse endothelial cells. (C) CD4+NK1.1+-activated T cells; αβTCR+CD4−CD8− resting T cells; αβTCR+CD4−CD8− -activated T cells; pro-T-resting cells; and pro-T-activated cells.
In addition, mCXCR3 also was detected in a B cell cDNA library (Fig. 2B), in line with a recent report that found chemotactic activity of 6Ckine for B cells (13, 19). CXCR3 expression also was detected in endothelial cells (Fig. 2B).
6Ckine Binds to CXCR3.
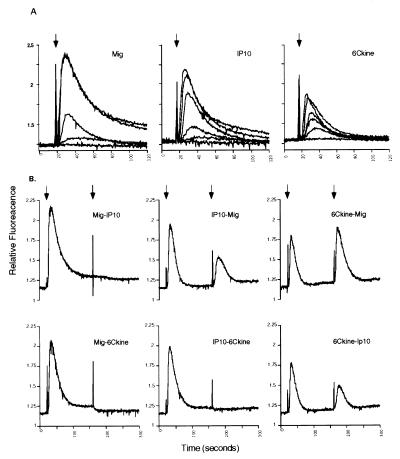
To study calcium mobilization, we generated stable transfectants in HEK293 cells with p-flag-CMV3-mCXCR3. As reported for hCXCR3, the CXC chemokines Mig and IP10 induced calcium flux in the CXCR3 transfectants, whereas nontransfected HEK293 did not show this response (Fig. 3A). Mig and IP-10 induced rapid receptor desensitization after successive treatment with the respective chemokines (Fig. 3B). We then tested 15 ligands, including many recently described chemokines that had not been tested previously against CXCR3 transfectants (Table 1). Surprisingly, the recently described CC chemokine 6Ckine also induced a Ca2+ flux in the CXCR3-transfected cells. Cross-desensitization experiments showed that Mig and IP-10 desensitize the 6Ckine response; however, the reverse desensitization pattern induced only partial desensitization. This phenomenon has been documented between IP-10 and Mig (9). Taken together, these data suggest that the affinity or binding avidity of CXCR3 is highest for Mig and lower for IP-10 and 6Ckine or that these ligands interact with CXCR3 through different binding sites.
Figure 3.
Calcium flux and desensitization analysis. (A) Calcium mobilization and dose response of Mig, IP-10, and 6Ckine with mouse CXCR3 stable-transfected HEK293 cells. Arrow indicates the time point of addition of the indicated ligands. Each chemokine was loaded from 1 nmol to 1 μmol in concentration. (B) Cross desensitization among Mig, IP-10, and 6Ckine. Arrows indicate the first and the second addition of the indicated chemokines. The chemokines were loaded at 1 μmol in concentration to induce a maximum calcium mobilization response.
6Ckine Exhibits Angiostatic Activity.
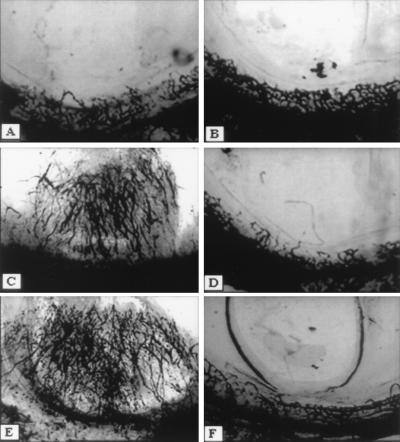
Because two other known CXCR3 ligands (Mig and IP-10) exhibit angiostatic activity, we decided to examine the potential angiostatic activity of 6ckine in vivo. To this end, hydron pellets alone, pellets containing 6Ckine (10 nM), bFGF (5 nM), or VEGF (5 nM) or pellets containing combinations of 6Ckine + bFGF or 6Ckine + VEGF, were embedded into the normally avascular rat cornea and assessed for a neovascular response (Fig. 4). Both bFGF and VEGF induced positive corneal angiogenic responses in four of four corneas tested, without evidence for significant leukocyte infiltration (assessed by light microscopy) (Table 2). In contrast, hydron pellets alone (n = 4 corneas), or pellets containing 6Ckine (n = 12 corneas for each chemokine), only resulted in a positive neovascular response in 17% of the corneas tested for each variable. When 6Ckine was added in combination with bFGF or VEGF (Fig. 4 and Table 2, respectively), it inhibited either the bFGF or VEGF-induced angiogenesis in five of six corneas (n = 6 corneas for each manipulation). These results indicate that 6Ckine has angiostatic activity.
Figure 4.
Angiostatic activity of 6Ckine. Angiogenic response in the rat corneal micropocket assay to bFGF (C) (5 nM) and VEGF (E) (5 nM) were inhibited by 6Ckine (10 nM) (D and F, respectively). Control (A) was DMEM with 0.1% BSA alone or 6Ckine alone (B).
Table 2.
The angiogenic response of 6Ckine, bFGF, VEGF, or in combination using the rat cornea micropocket assay for neovascularization
| Condition | Corneas positive for neovacularization (percentage) |
|---|---|
| Control (vehicle alone) | 0 of 4 (0%) |
| bFGF (5 nM) | 4 of 4 (100%) |
| VEGF (5 nM) | 4 of 4 (100%) |
| 6Ckine (10 nM) | 2 of 12 (17%) |
| bFGF (5 nM) + 6CKine (10 nM) | 1 of 6 (17%) |
| VEGF (5 nM) + 6CKine (10 nM) | 1 of 6 (17%) |
CXCR3 Maps to Chromosome X.
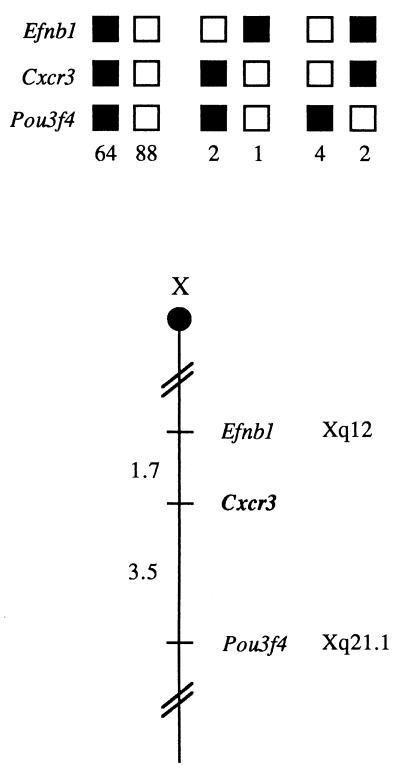
The mouse chromosomal location of Cxcr3 was determined by interspecific backcross analysis by using progeny derived from mating of [(C57BL/6J × M. spretus)F1 × C57BL/6J] mice (16). C57BL/6J and M. spretus DNAs were digested with several enzymes and analyzed by Southern blot hybridization for restriction fragment length polymorphisms by using a CXCR3 cDNA probe. The 7.8-kb EcoRI M. spretus restriction fragment length polymorphism was used to follow the segregation of the Cxcr3 locus in backcross mice. The mapping results indicate that Cxcr3 is located in the central region of the mouse X chromosome linked to Efnb1 and Pou3f4. (Fig. 5). The ratios of the total number of mice exhibiting recombinant chromosomes to the total number of mice analyzed for each pair of loci and the most likely gene order are: centromere–Efnb1–3/172–Cxcr3–6/174–Pou3f4. The recombination frequencies [expressed as genetic distances in centimorgans (cM) ± the standard error] are –Efnbl–1.7 ± Cxcr3–3.5 ± 1.4–Pou3f4.
Figure 5.
Cxcr3 maps to the central region of the mouse chromosome X. Cxcr3 was placed on the mouse chromosome X by interspecific backcross analysis. The segregation patterns of Cxcr3 and flanking genes in 161 backcross animals that were typed (see text). Each column represents the chromosome identified in the backcross progeny that was inherited from the (C57BL/6J × M. spretus)F1 parent. The shaded boxes represent the presence of a C57BL/6J allele, and white boxes represent the presence of a M. spretus allele. The number of offspring inheriting each type of chromosome is listed at the bottom of each column. A partial X chromosome-linkage map showing the location of Cxcr3 in relation to linked genes is shown at the bottom of the figure. Recombination distances between loci in centimorgans are shown to the left of the chromosome and the positions of the loci in human chromosomes, where known, are show to the right. References for the human map positions of loci cited in this study can be obtained from Genome Database (GDB), a computerized database of human linkage information maintained by the William H. Welcher Medical Library of The Johns Hopkins University (Baltimore, MD).
We have compared our interspecific map of the X chromosome with a composite mouse linkage map that reports the map location of many uncloned mouse mutations (provided from the Mouse Genome Database, a computerized database maintained at the Jackson Laboratory, Bar Harbor, ME). Cxcr3 maps in a region of the composite map that lacks mouse mutations with a phenotype consistent with an alteration in this locus (data not shown).
The corresponding syntenic human region is located in chromosome X. However, this prediction was not consistent with a previous report in which human CXCR3 (GPR9) was mapped by fluorescence in situ hybridization to chromosome 8 (20). To clarify this, we performed PCR analysis by using human CXCR3 primers under high stringency conditions on hamster/human hybrid lines. The results showed a specific band in hybrids containing human chromosome X (data not shown), confirming that human Cxcr3 is located in human chromosome X.
DISCUSSION
Chemokines have been classified in four classes, CXC, CC, C, and CX3C, and the chemokines receptors have been subdivided on the basis of their ligands into CXCR and CCR. So far the chemokine receptors described only bind ligands from a single chemokine class (CXC or CC), with the only exception being the Duffy antigen/receptor for chemokines (DARC), which does not induce a calcium flux but binds certain CC and CXC chemokines (6). Recently, the discovery of new chemokines has accelerated by using genomic and bioinformatics-based searches (14). This way a CC chemokine (6Ckine) was identified (5, 12, 19). While screening for new chemokine receptors in a cDNA library from αβTCR+CD4−CD8− thymocytes, we isolated a cDNA encoding the mouse homologue of CXCR3. We then observed that 6Ckine is a ligand for CXCR3. Given that 6Ckine is a CC chemokine, this represents an example of a CC chemokine binding a CXC receptor. This is a particular feature of 6Ckine because none of the other CC ligands tested bind CXCR3 (Table 2). Recently, 6ckine also has been shown to bind CCR7 (21, 22) but not other CCRs tested (CCR1–CCR6) (22). We have observed that CCR7 expression in T cells is either very low or absent (data not shown). In contrast, we found significant expression of CXCR3 in various T cells populations (Fig. 2). The latter observation suggests that the chemotactic effects of 6ckine in T cells are mainly mediated through CXCR3. In fact, the other two chemokines known to bind CXCR3, Mig and IP-10, are known to preferentially affect T cells (23).
The high expression of CXCR3 in Rag-1−/− thymus suggests that this receptor is expressed by thymic progenitors or stromal cells and therefore could be involved in T cell development. It has been suggested that hCXCR3 mediates selective lymphocyte recruitment (9); these data correlate with mCXCR3 distribution in CD4+-derived cells. Human CXCR3 is not expressed in macrophages (9), but one of its known ligands, IP-10, has been reported to be a chemoattractant for both macrophages and T lymphocytes. It was recently described that hCXCR3 is highly expressed in 12 days polarized Th1 but not in Th2 cells (18). We obtained similar results for mCXCR3 by Northern and Southern analyses (Fig. 2D) and also observed that the difference in mCXCR3 mRNA expression between Th1- and Th2-polarized cells increases after 3 wk (Fig. 2). We therefore analyzed the expression of CXCR3 in some models of mouse inflammatory disease, and found it up-regulated in the mesenteric lymph nodes and Peyer’s patches of IL-10−/− mice, supporting the conclusion that Th1 cells play a role in the development of enterocolitis in IL-10−/− mice (24, 25). Recently, 6Ckine has been shown to be produced by vascular endothelium (13) and able to favor the initial binding of circulating cells with the endothelium (26). Taken together, these observations strongly suggest a critical role for 6Ckine in the recruitment of T cells and other leukocytes from the circulation.
If 6Ckine binds CXCR3, it should share biological activities with IP-10 and Mig. Other chemokines have suppressive effects in hematopoietic progenitors (27), and 6Ckine also exhibits this characteristic (12). Our results suggest that the inhibitory effect of 6Ckine may be mediated by CXCR3 as well.
The binding of 6Ckine to CXCR3 suggested that it could have angiostatic effects, as has been reported for IP-10 and Mig (28, 29). Previous work showed that IP-10 and Mig inhibit angiogenesis induced by IL-8 or by bFGF in the rat corneal micropocket assay and that IP-10 or Mig block the endothelial cell chemotaxis to IL-8 or to bFGF (29). To determine whether 6Ckine could inhibit the angiogenic activity of bFGF or VEGF in vivo, 6Ckine was used in the rat corneal micropocket assay. As predicted, 6Ckine showed a strong angiostatic effect (Fig. 4). This represents an example of a CC chemokine with angiostatic properties. The ELR-CXC chemokines favor angiogenesis, unlike the non-ELR-CXC chemokines (PF-4, IP-10, and Mig), which are angiostatic (29). It also is known that the ELR motif in CXC chemokines has a functional role in chemokine-mediated angiogenesis (29). By analogy, it is likely that the AQD motif before the first two cysteines of 6Ckine may be connected with the angiostatic properties of 6Ckine, as has been demonstrated for the TVR and DLQ motifs present in IP-10 and PF4, respectively (29).
The binding of IP-10 and Mig to CXCR3 has not been studied from the structural point of view. Structural studies with IL-8 and GROα showed that the flexible NH2-terminal region is the most critical receptor binding site for these CXC chemokines, and that a second binding site exists in the loop that follows the two disulfides (30). Whether any of these conclusions applies to the binding of 6Ckine to CXCR3 remains to be determined.
The ability of 6Ckine to chemoattract T cells, including Th1 cells, and its angiostatic activity make it a strong candidate to test in cancer immunotherapy. IP-10 is known to be expressed, along with IL-6 and TNFα, at higher levels by regressing tumors, compared with progressing tumors (31). Experiments to demonstrate the IP-10 antitumor response were performed by using genetically engineered tumor cells that secrete high levels of IP-10. Although this chemokine did not have adverse effects on the growth of these tumor cell cultures, it elicited a powerful host-mediated antitumor effect in vivo, which is T cell dependent (32, 33). Similarly, Mig has antitumor activity in vivo (34). The antitumor effect of IP-10 is also evident in SCID mice, suggesting that these chemokines also attenuate tumor growth through inhibition of angiogenesis (35). Taken together, these observations strongly suggest that 6Ckine has antitumor activity like the other CXCR3 ligands.
We conclude that the angiostatic activity of 6Ckine is likely to be mediated through its interaction with CXCR3. Originally, it was described that a specific cell surface heparan sulfate proteoglycan receptor on endothelial cells, which binds IP-10 and PF4 (36), could be the potential endothelial receptor for CXC angiostatic chemokines. Nevertheless, it is not known whether this receptor represents CXCR3. It has suggested that the heparan sulfate containing proteoglycan and CXCR3 are the same molecule (1). In vitro and in vivo systems have demonstrated (35) that the CXC chemokines behave as either angiogenic or angiostatic factors, depending on the presence of the ELR motif. In addition, it has been suggested that IP-10 and Mig may be distal mediators of the angiostatic effects of interferons (35, 34). It remains to be determined whether 6Ckine expression is up-regulated by IFNγ.
GPCR genes are distributed throughout the human genome (37). Certain chemokine receptors are genetically clustered. The CC receptors (CCR1–5, CCR8) are located in human chromosome 3 (38), hCCR6 gene maps to chromosome 6 (39); whereas CXCR1, CXCR2, and CXCR4 are clustered on human chromosome 2. We found that mouse and human CXCR3 map to chromosome X, representing a new locus for chemokine receptors. Nevertheless, it is not the only one GPCR that maps in that chromosome because several opsin (photoreceptor proteins) genes are clustered on the X chromosome (37). The central region of the mouse X chromosome is syntenic with a region in the long arm of the human X chromosome (summarized in Fig. 5), suggesting that the human homologue of Cxcr3 maps to Xq, as well.
The experiments described here point to 6Ckine as a potential anti-tumor agent. Furthermore, we showed that 6Ckine is an example of a CC chemokine binding a CXC chemokine receptor. Future experiments will aim to investigate these possibilities.
Acknowledgments
We thank Deborah B. Householder for excellent technical assistance and Terri McClanahan for providing cDNA libraries. This research was supported, in part, by the National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratories. DNAX Research Institute is supported by Schering-Plough Corporation. This work was supported by a grant from National Institutes of Health (CA66180) to R.M.S.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; GPCR, G protein-coupled receptors; TMD transmembrane domain.
Data deposition: The mouse CXCR3 sequence reported in this paper has been deposited in the GenBank database (accession no. AF045146).
References
- 1.Rollins B J. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Kelner G, Kennedy J, Bacon K, Kleyensteuber S, Largaespada D, Jenkins N, Copeland N, Bazan J F, Moore K, Schall T J, et al. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 4.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greavbes D R, Zlotnik A, Schall T J. Nature (London) 1997;385:640–664. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 5.Hedrick J A, Zlotnik A. J Immunol. 1997;159:1589–1593. [PubMed] [Google Scholar]
- 6.Murphy P M. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 7.Holmes W E, Lee J, Kuan G, Rice C, Wood W I. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 8.Van Damme J, Wuyts A, Froyen G, Van Coillie E, Struyf S, Billiau A, Proost P, Wang J M, Opdenakker G. J Leukocyte Biol. 1997;62:563–569. doi: 10.1002/jlb.62.5.563. [DOI] [PubMed] [Google Scholar]
- 9.Loetscher M, Grever B, Loetscher P, Jones S A, Piali L, Clark-Lewis I, Baggiolini M, Moser B. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodrosky J, Springer T. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 11.Legler D F, Loetscher M, Roos R S, Clark-Lewis I, Baggiolini M, Moser B. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hromas T, Kim C H, Klemsz M, Krathwohl M, Fife K, Cooper S, Schnizlein-Bick C, Broxmeyer H E. J Immunol. 1997;159:2554–2558. [PubMed] [Google Scholar]
- 13.Gunn M D, Tagemann K, Tam C, Cyster J G, Rosen S D, Williams L T. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi D, Vicari A, Frinz-Bacon K, McClanahan T, Zlotnik A. J Immunol. 1997;158:1033–1036. [PubMed] [Google Scholar]
- 15.Arenberg D A, Kunkel S, Polverini P J, Morris S B, Burdick M D, Glass M C, Taub D T, Iannettoni M D, Whyte R I, Strieter R M. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copeland N G, Jenkins N A. Trends Genet. 1991;7:113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- 17.Avraham B B, Cho B C, Gilbert D J, Fijii H, Okamoto K, Shimazaki T, Ito T, Shoji H, Wakamatsu Y, Kondoh H, et al. Genomics. 1993;18:131–133. doi: 10.1006/geno.1993.1436. [DOI] [PubMed] [Google Scholar]
- 18.Bonecchi R, Bianchi G, Bordignon P P, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Avellana P, Gray A P, Mantovani A, et al. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagira M, Imai T, Hieshima K, Kusuda J, Ridanpaa M, Takagi S, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. J Biol Chem. 1997;272:19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- 20.Marchese A, Docherty J M, Nguyen T, Heiber M, Cheng R, Heng H H, Tsui L C, Shi X, George S R, O’Dowd B F. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- 21.Campbell J, Bowman E, Murphy K, Youngman K, Siani M, Thompson D, Wu L, Zlotnik A, Butcher E. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida R, Nagira M, Kitaura M, Imagawa N, Imai T, Yoshie O. J Biol Chem. 1998;273:7118–7122. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- 23.Farber J M. J Leukocyte Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 24.Rennick D M, Fort M M, Davidson J. J Leukocyte Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 25.Wynn T A, Morawetz R, Scharton-Kersten T, Hieny S, Morse H C I, Kuhm R, Muller W, Cheever A W, Sher A. J Immunol. 1997;159:5014–5023. [PubMed] [Google Scholar]
- 26.Campbell J J, Hedrick J, Zlotnik A, Siani M A, Thompson D A, Butcher E C. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 27.Broxmeyer H, Cooper S, Hague N, Benninger L, Sarris A, Cornetta K, Vadham-Raj S, Hendrie P, Mantel C. Annu Rev Hematol. 1995;71:235–246. doi: 10.1007/BF01744373. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo A L, Sgadari C, Taub D D, Liao F, Farber J M, Maheshwari S, Kleinman H K, Reaman G H, Tosato G. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strieter R, Polverini P J, Kunkel S, Arenberg D A, Burdick M, Kasper J, Dzuiba J, VanDammer J, Walz A, Marriott D, et al. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 30.Clark-Lewis I, Soo K, Ragarathnam K, Gong J, Dewalt B, Moser B, Baggiolini M, Sykes B. J Leukocyte Biol. 1995;57:703–711. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 31.Tosato H, M, Sgadari C, Taga K, Jones K, Pike S, Rosenberg A, Sechler J, Magrath I, Love L, Bhatia K. Blood. 1994;83:776–784. [PubMed] [Google Scholar]
- 32.Luster A D, Leder P. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgadari C, Angiolillo A, Cherney B, Pike S, Farber J, Koniaris L, Vanguri P, Brud P, Sheikh N, Gupta G, et al. Proc Natl Acad Sci USA. 1996;93:13791–13796. doi: 10.1073/pnas.93.24.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgadari C, Farber J, Anguillo A, Liao F, Teruya-Feldstein J, Burd P, Yao L, Gupta G, Kanegane C, Tosato G. Blood. 1997;89:2635–2643. [PubMed] [Google Scholar]
- 35.Arenberg D A, Polverini P J, Kunkel S L, Shanafelt A, Hesselgesser J, Horuk R, Strieter R M. J Leukocyte Biol. 1997;62:554–562. doi: 10.1002/jlb.62.5.554. [DOI] [PubMed] [Google Scholar]
- 36.Luster A, Greenberg S, Leder P. J Exp Med. 1995;182:219–232. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 38.Bonini J, Martin S, Dralyuk F, Roe M, Philipson L, Steiner D. DNA Cell Biol. 1997;16:1249–1256. doi: 10.1089/dna.1997.16.1249. [DOI] [PubMed] [Google Scholar]
- 39.Greaves D, Wang W, Dairaghi D, Dieu M, de Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, et al. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]