Abstract
Aim
To evaluate the effectiveness of 2.5% povidone‐iodine eye drops (PIED) compared with ophthalmic chloramphenicol (OC) for preventing neonatal conjunctivitis.
Methods
2004 neonates were enrolled from three rural hospitals in a trachoma endemic area. They were randomly assigned to receive either PIED (n = 1024) or OC (n = 974). Infectious conjunctivitis was confirmed by laboratory methods, including specific search for Chlamydia trachomatis by polymerase chain reaction assay.
Results
During the first 48 hours after birth, PIED and OC had similar efficacy against bacterial conjunctivitis (95% confidence interval (CI), −0.031 to −0.004; p = 0.01); from day 3 to day 15, PIED was 6% less effective than OC (95% CI, −0.058 to −0.006; p = 0.01); after day 16 there was no significant difference between the groups (95% CI, −0.022 to 0.041; p = 0.57). However, the risk of C trachomatis conjunctivitis was increased in neonates receiving PIED prophylaxis (relative risk = 1.99 (95% CI, 1.07 to 3.71), log‐rank p = 0.029). Ocular side effects were rare and self limiting in both groups (p = 0.223).
Conclusions
PIED seems to increase the risk of acquiring chlamydial conjunctivitis in neonates. Additional measures are required to prevent mother to fetus transmission of chlamydial infection during pregnancy, delivery, and after birth.
Keywords: ophthalmia neonatorum, Chlamydia trachomatis , trachoma, blindness
Neonatal conjunctivitis is defined as an external ocular infection occurring during the first month of life. It represents a public health problem in developing countries as it can cause irreversible blindness.1,2 According to our current health policy statements, eye prophylaxis in neonates is a legal requirement in Mexico.3 During the last 20 years, this procedure has consisted of the application of a single drop of ophthalmic chloramphenicol (OC) in both eyes shortly after birth. Although chloramphenicol is a broad spectrum antibiotic, its indiscriminate use in Mexico has raised the question of its effectiveness against bacteria that may cause blindness, such as Neisseria gonorrhoeae and Chlamydia trachomatis. OC effectiveness in the prevention of neonatal conjunctivitis has never been clinically tested in Mexico. Isenberg and collaborators have shown that the use of 2.5% povidone‐iodine eye drops (PIED) was effective in the prevention of conjunctival infections in two different clinical settings.4,5 With the emergence of chlamydia as a major cause of neonatal conjunctivitis, there is a need to seek alternative methods of prophylaxis that are effective against this disease.
Methods
The study was designed in accordance with the Declaration of Helsinki. Ethics approval for the study was obtained from the Hospital Infantil de Mexico Committee on Human Research and from the ethics committee of attending hospitals.
Patients were enrolled from three rural hospitals in the highlands of Chiapas, a marginalised area and well known for active trachoma according to the World Health Organisation.6 All neonates born by vaginal delivery or caesarean section were included. Patients were excluded if they had eyelid malformations that prevented appropriate conjunctival evaluation or in the case of death during the first month of life. Mothers were instructed to return to the hospital in case of possible symptoms of neonatal conjunctivitis, illustrated by pictures of infected eyes before the infant left hospital. Parents were also requested to return to the hospital at any time if there was redness or discharge in their child's eyes.
Ocular examinations were always carried out by general practitioners trained in the procedure. After delivery, all infants' eyes were examined in the first 24 to 48 hours in the postnatal ward. A second eye examination was scheduled between day 10 and day 15, and a third between day 16 and day 30. When eye examinations were done at the parents' request, the infants were assigned to the second or third eye examination group according to the day of their hospital visit. When parents did not take their infants back to hospital, a field team visited their registered address. Neonatal conjunctivitis was defined clinically by a yellow or greenish discharge in the conjunctival cul‐de‐sac or involving the eyelids and eyelashes, or both. All neonates with suspected neonatal conjunctivitis were treated with topical antibiotics after conjunctival samples had been taken. The babies were also evaluated for side effects of prophylactic treatment during the first 24 to 48 hours—particularly non‐infectious conjunctivitis (conjunctival hyperaemia, chemosis, and eyelid swelling without greenish or yellowish discharge). Serious adverse reactions such as bronchospasm or death were reported to the study coordinator within 24 hours.
Neonates were randomly assigned to receive PIED or OC eye drops. Eye prophylaxis was carried out by nursing personnel within 20 minutes of birth. The eyelids were wiped immediately after prophylaxis. Randomisation assignments were allocated centrally in a weekly fashion by the coordinating centre in a 1: 1 ratio.
Two conjunctival samples were taken for bacterial cultures when eye infection was suspected. The first swab was inoculated on to blood agar enriched with 5% sheep blood, MacConkey agar, mannitol salt agar, and chocolate agar. Cultures were analysed daily following the American Society for Microbiology guidelines.7 Colonies suspected to be pathogens were selected and investigated by Gram stain. Depending on the results of the Gram stain, a second sample was obtained for the diagnosis of chlamydia by polymerase chain reaction (PCR). The upper tarsal plates were swabbed with a sterile Dacron swab passed across the tarsal conjunctiva four times, with the swab rotated a quarter turn with each pass. Each swab was placed in 1 ml of sucrose phosphate (2SP) transport medium supplemented with antibiotics, and stored at −17°C until shipped to the laboratory for processing. Chlamydial DNA was extracted by phenol‐chloroform‐isoamyl alcohol protocol (25:24:1). DNA was precipitated with ethyl alcohol and solubilised with ultrapure water. The Omp1 gene was amplified by PCR as described elsewhere.8 Amplification was carried out on a Perking‐Elmer Gene Amp PCR system 2400 (Irvine, California, USA). Random sample swabs were also taken in asymptomatic infants as controls.
Statistical analysis
The primary analysis of the event was based on a per protocol analysis. Patients were grouped according to the prophylaxis used. Bivariate analysis with a z test of proportions was used to compare differences between proportions in the two groups. Student's t test was applied to determine differences between means. A Cox proportional hazards model was developed to determine the influence of the baseline covariates on the differences between the groups, adjusting for risk factors such as sex, mode of delivery, premature rupture of membranes, urinary tract infections, prenatal care, and household characteristics, considering neonatal conjunctivitis as a dependent variable. The period of development of the disease was estimated using the cumulative incidence analysis and the relations between identification of cultured organisms. PCR status and time of disease in both groups were compared using the Mantel–Cox log‐rank test, with patient follow up censored at the time of the event or on the last follow up date. Probability (p) values were considered significant at p<0.05. Statistical analyses were conducted using SAS (SAS Institute, Cary, North Carolina, USA) and Stata 7.0 (Stata Corporation, College Station, Texas, USA).
Sample size was calculated on the basis of clinical equivalence9 with an α level of 0.05 (two tailed), a power of 0.80, and an estimated neonatal conjunctivitis incidence of 13% (based on a previous unpublished study). A total sample size of 660 patients per group was calculated. Allowing for an estimated loss of 35% of cases after the start of the study, 270 additional infants were recruited. PIED would be judged as effective as OC if the lower and upper limits came with respect of the two sided 95% confidence interval for the difference in response rates and had to be entirely in the prespecified equivalence threshold of 3%.
Results
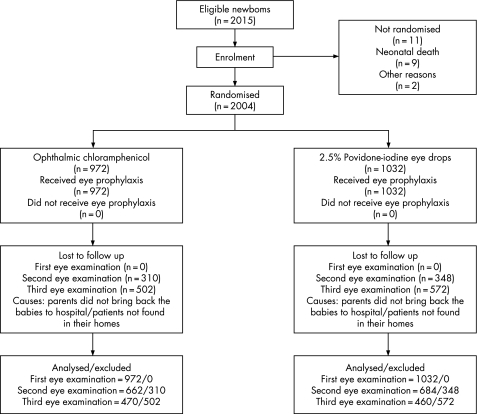
We recruited 2004 infants, with 972 randomised to OC and 1032 to PIED. Reasons for failure of follow up are given in fig 1. The risk factor distribution in the two treatment groups was homogeneous and no significant differences were found (table 1). The most frequently isolated bacteria were coagulase negative staphylococci in 136 patients (72.3%), followed by Chlamydia trachomatis in 46 (24.5%). The risk of developing coagulase negative staphylococcal infections was not significantly different between the two treatment groups during the follow up period (relative risk (RR) = 1.39 (95% confidence interval (CI), 0.98 to 1.99), p = 0.06). There were no cases of gonococcal conjunctivitis (table 2).
Figure 1 Participation flow chart and randomisation assignment of neonates receiving eye prophylaxis against neonatal conjunctivitis.
Table 1 Baseline characteristics of the infants by treatment group.
| Chloramphenicol n = 972 | Povidone‐iodine n = 1032 | ||||
|---|---|---|---|---|---|
| Cases (n (%)) | Mean (SD) | Cases (n (%)) | Mean (SD) | p Value | |
| Newborn | |||||
| Male sex | 508 (51.7) | 500 (48.4) | 0.14 | ||
| Birth weight (g) | 3033.0 (458.7) | 3042.8 (490.3) | 0.64 | ||
| Maternal health care history | |||||
| Prenatal care visits | 5.31 (2.86) | 5.55 (3.02) | 0.07 | ||
| Premature rupture of membranes | 328 (33.7) | 298 (28.9) | 0.02* | ||
| Urinary tract infection | 165 (17.0) | 192 (18.6) | 0.34 | ||
| Mode of delivery | |||||
| Caesarean section | 244 (25.3) | 271 (26.4) | 0.57 | ||
| Sociodemographic characteristics | |||||
| Rural household | 191 (19.7) | 201 (19.5) | 0.91 | ||
| Dirty floor | 366 (37.7) | 367 (35.6) | 0.32 | ||
| Households with available running water | 839 (86.3) | 900 (87.2) | 0.55 | ||
| Tzotzil/Tzeltal spoken as mother tongue | 499 (51.3) | 509 (49.4) | 0.39 | ||
| Reference hospital | |||||
| Rural, San Felipe Ecatepec | 306 (31.5) | 361 (35.0) | 0.09 | ||
| General, San Cristóbal de las Casas | 345 (35.5) | 339 (32.8) | 0.2 | ||
| Rural, Ocosingo | 321 (33.0) | 332 (32.2) | 0.7 | ||
| Follow up | 18.54 (13.11) | 17.59 (13.07) | 0.11 | ||
*Cox regression model did not show differences by treatment group after adjusting for premature rupture of membranes.
Table 2 Incidence density per 1000 neonate days of bacteria isolated from conjunctival specimens by treatment group.
| Chloramphenicol | Povidone‐iodine | RR | 95% CI | p Value† | |||
|---|---|---|---|---|---|---|---|
| Cases/neonate days | ID | Cases/neonate days | ID | ||||
| Coagulase‐negative staphylococcus | 58/17 532 | 3.3 | 78/16 999 | 4.6 | 1.39 | 0.98 to 1.99 | 0.06 |
| Chlamydia trachomatis | 16/17 532 | 0.91 | 30/16 999 | 1.76 | 1.93 | 1.02 to 3.80 | 0.03 |
| Enterobacteriae* | 12/17 532 | 0.68 | 18/16 999 | 1.05 | 1.55 | 0.71 to 3.52 | 0.24 |
| Staph aureus | 12/17 532 | 0.68 | 14/16 999 | 0.82 | 1.20 | 0.52 to 2.85 | 0.64 |
| Staph epidermidis | 7/17 532 | 0.39 | 15/16 999 | 0.88 | 2.21 | 0.84 to 6.41 | 0.08 |
| Candida spp | 3/17 532 | 0.17 | 7/16 999 | 0.41 | 2.41 | 0.55 to 14.42 | 0.18 |
| Streptococci | 3/17 532 | 0.17 | 4/16 999 | 0.23 | 1.37 | 0.23 to 9.39 | 0.69 |
| Positive cultures | 77/17 532 | 4.4 | 111/16 999 | 6.5 | 1.49 | 1.1 to 2.05 | 0.007 |
| No growth | 4 | 8 | |||||
Data are shown by frequency. Some patients were infected by more than one bacterium.
*E coli, Klebsiella spp, Pseudomonas spp, Aeromona spp, enterobacter.
†Pearson's χ2.
CI, confidence interval; ID, incidence density.
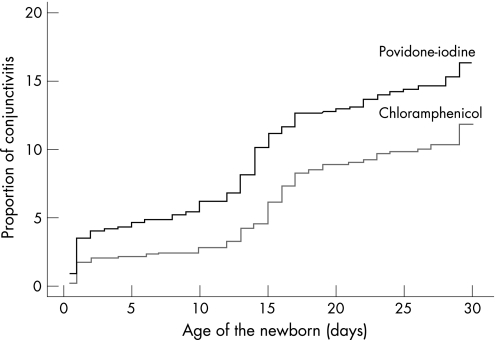
During the first 48 hours, the difference in effectiveness of PIED vs OC against bacterial conjunctivitis was no greater than 3% (95% CI, −0.031 to −0.004; p = 0.01). From day 3 to day 15 the difference broadened to almost 6% (95% CI, −0.058 to −0.006; p = 0.01). After day 16 there was no significant difference in the incidence of bacterial conjunctivitis between the groups (95% CI, −0.022 to 0.041; p = 0.57). Overall, there was a greater risk of developing infectious conjunctivitis in the PIED group (RR = 1.55 (95% CI, 1.17 to 2.04), log‐rank p = 0.002) (fig 2).
Figure 2 Cumulative incidence of overall infectious neonatal conjunctivitis confirmed in laboratory in the povidone‐iodine eye drops (PIED) and ophthalmic chloramphenicol groups during the first 30 days of life, showing increased risk of eye infection in infants receiving PIED prophylaxis (log rank, p = 0.002).
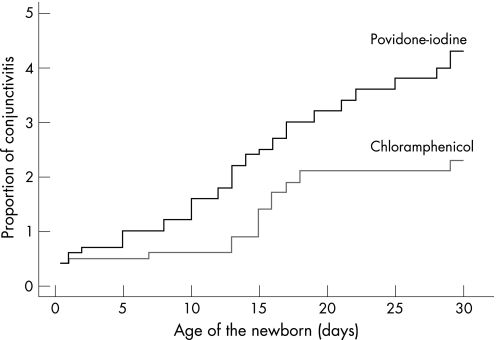
Determination of cumulative incidence rates also showed an increased risk of developing C trachomatis conjunctivitis in the PIED group (RR = 1.99 (95% CI, 1.07 to 3.71), log‐rank p = 0.029) (fig 3). There were no significant differences in mode of delivery or chlamydial eye infections between the two groups. One infant delivered by caesarean section in the PIED group was found to be positive to C trachomatis during the first 48 hours. There was no significant difference in the number of patients infected with chlamydia strains belonged to trachoma serovar C (table 3).
Figure 3 Cumulative incidence of neonatal conjunctivitis secondary to Chlamydia trachomatis in the povidone‐iodine eye drops (PIED) and ophthalmic chloramphenicol groups during the first 30 days of life, showing increased risk of chlamydial eye infections in infants receiving PIED prophylaxis (log rank, p = 0.029).
Table 3 Detection of Chlamydia trachomatis serotypes by polymerase chain reaction in 169 eye specimens taken from neonates with a clinical suspicion of infectious conjunctivitis during three different eye examinations.
| Mode of delivery | Prophylaxis (samples/patients) | Serotype | Number of cases | Total | ||
|---|---|---|---|---|---|---|
| First | Second | Third | ||||
| examination | examination | examination | ||||
| Normal vaginal delivery | Chloramphenicol (55/728) | C | 1 | 2 | 2 | 5 |
| D, E, F | 2 | 2 | 2 | 6 | ||
| Not available* | 1 | 1 | ||||
| Total | 3 | 4 | 5 | 12 | ||
| Povidone‐iodine (70/761) | C | 1 | 2 | 3 | 6 | |
| D, E, F | 4 | 8 | 3 | 15 | ||
| Not available* | 1 | 3 | 4 | |||
| Total | 5 | 11 | 9 | 25 | ||
| Caesarean section | Chloramphenicol (18/244) | C | 2 | 2 | ||
| D, E, F | 2 | 2 | ||||
| Total | 4 | 4 | ||||
| Povidone‐iodine (26/271) | C | 2 | 2 | |||
| D, E, F | 1 | 1 | 1 | 3 | ||
| Total | 1 | 3 | 1 | 5 | ||
*The DNA material obtained was not sufficient to undertake restriction fragment length polymorphism analysis (RFLP).
Ocular side effects were rare and self limiting in both groups: seven of the 972 children who received OC and 13 of the 1032 who received PIED developed non‐infectious conjunctivitis attributable to the use of the prophylactic solution (p = 0.223).
Discussion
This randomised trial showed no benefit of either PIED or OC prophylaxis in reducing chlamydial conjunctivitis in neonates. However, we found a greater risk of acquiring chlamydial eye infections in those receiving PIED. This may be explained by changes in conjunctival bacterial flora following the use of a broad spectrum antimicrobial agent. Another possible explanation is that the effectiveness of PIED may decrease because of a change in its bactericidal properties during its manufacture and storage, or because of unidentified environmental factors specific to the region where it was used. Although PIED has been widely used to reduce the risk of infection, some studies have failed to demonstrate its efficacy in controlling particular eye infections.10,11
There are three possible routes of chlamydial transmission to neonates: prenatal transmission through the amniotic fluid,12,13 perinatal transmission from the maternal genital tract, and postnatal transmission (eye to eye, hand to eye, or saliva to eye). Previous studies have not demonstrated increased perinatal transmission of C trachomatis in areas of high trachoma incidence.14,15 We found nine infants in whom C trachomatis was isolated during the first 48 hours of life. One of these was born by caesarean section from a multiparous mother with a history of prenatal urinary infection. There were 15 infants who were positive to trachoma serotype C. However, the value of this finding is limited because we did not take samples from the cervices or throats of the mothers of infected infants.
There were no differences in the prevention of eye infections caused by coagulase negative staphylococci between the two groups. Coagulase negative staphylococci are the most common bacteria isolated in cases of neonatal conjunctivitis and can be acquired from the maternal flora and from surrounding environmental sources. We did not find any cases of neisseria induced ophthalmia neonatorum in over 2000 births following ocular prophylaxis.
One limitation of our study was the loss of follow up after the third eye examination. It is therefore possible that the incidence of neonatal conjunctivitis after day 16 was underestimated. However, the great majority of the cases were diagnosed during hospital visits, as most of the positive bacterial isolates were obtained during the first 15 days of life. Despite all efforts made by the field team to locate the families who did not come back for their third hospital appointment, many children could not be found at their registered address to rule out eye infection. Most of these came from poorer families living in remote places with extremely difficult road access to some communities. The small proportion of patients with eye infections found at their addresses probably reflected the success of the instructions given to the mothers during their hospital stay. Nevertheless caution is needed in interpreting these data because infants living in an unhygienic environment are at an increased risk of acquiring conjunctivitis. Chlamydial infection may remain asymptomatic for long periods, so a four week follow up could result in an underestimation of the actual number of infants with chlamydial conjunctivitis, as has been stated before.16
In conclusion, the prevention of neonatal conjunctivitis is a complex task which cannot be achieved by a single mandatory prophylaxis. Several prospective studies have shown that mother to infant transmission of C trachomatis cannot be completely eliminated with the most common antimicrobial agents currently prescribed for preventing neonatal conjunctivitis, such as silver nitrate, erythromycin, or tetracycline ophthalmic ointment,17,18,19 neither is a double application approach capable of reducing the incidence.20 Environmental factors also influence the development of infectious conjunctivitis during the first 30 days of life. Given the apparent ineffectiveness of eye prophylaxis with PIED and OC in the prevention of chlamydial ophthalmia in southern Mexico, the most effective control method may be to screen and treat pregnant woman at high risk of chlamydial infection. Neonates at risk should be identified before delivery when the mother has a history of sexually transmitted disease, so that appropriate measures can be taken. It is also necessary to improve sociodemographic conditions, such as maternal health care availability, sexual education, and the eradication of poverty.
Acknowledgements
We thank Erik Suarez PhD from Puerto Rico University, Beatriz Munoz from Dana Center for Preventive Ophthalmology at Johns Hopkins University and Alfonso Reyes MSc from Research Department at Hospital Infantil de Mexico for their invaluable assistance in statistical analysis, and Marco A Leyva PhD, from Guerrero University for his assistance in PCR analysis.
Supported by Hospital Infantil de Mexico Board of Trustees Grant HIM/2002/024.
Abbreviations
OC - ophthalmic chloramphenicol
PIED - povidone‐iodine eye drops
Footnotes
Competing interests: None declared.
References
- 1.Klauss V, Schwartz E C. Other conditions of the outer eye. In: Johnson GJ, Minassian DC, Weale R, editors. The epidemiology of eye disease. London: Chapman and Hall, 1998
- 2.Klauss V, Fransen L. Neonatal ophthalmia in tropical countries. In: Bialasiewicz AA, Schaal KP, editors. Infectious diseases of the eye. London: Butterworth‐Heinemann, 1994
- 3.Secretaría de Salud pp. 19–38.
- 4.Isenberg S J, Apt L, Yoshimori R.et al Povidone‐iodine for ophthalmia neonatorum prophylaxis. Am J Ophthalmol 1994118701–706. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg S, Leonard A, Word M. A controlled trial of povidone‐iodine as prophylaxis against ophthalmia neonatorum. N Engl J Med 1995332562–566. [DOI] [PubMed] [Google Scholar]
- 6.Polack S, Brooker S, Kuper H.et al Mapping the global distribution of trachoma. Bull WHO . 2005; 83913–919. [PMC free article] [PubMed]
- 7.Hall G S, Pezzlo M. Ocular cultures. In: Isenberg HD, editor. Clinical microbiology procedures handbook. Washington DC: American Society for Microbiology, 1995
- 8.Yang B G, Maclean I, Brunham R C. DNA sequence polymorphism on the Chlamydia trachomatis Omp1 gene. J Infect Dis 19931681225–1230. [DOI] [PubMed] [Google Scholar]
- 9.Bristol D R. Clinical equivalence. J Biopharm Stat 19999549–561. [DOI] [PubMed] [Google Scholar]
- 10.Michalova K, Moyes A L, Cameron S.et al Povidone‐iodine (Betadine) in the treatment of experimental Pseudomonas aeruginosa keratitis. Cornea 199615533–536. [PubMed] [Google Scholar]
- 11.Katz J, Khatry S K, Thapa M D.et al A randomized trial of povidone‐iodine to reduce visual impairment from corneal ulcers in rural Nepal. Br J Ophthalmol 2004881487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas G B, Jones J, Sbarra A J.et al Isolation of Chlamydia trachomatis from amniotic fluid. Obstet Gynecol 199076519–520. [PubMed] [Google Scholar]
- 13.Djukic S, Nedeljkovic M, Pervulov M.et al Intra‐amniotic Chlamydia trachomatis infection. Gynecol Obstet Invest 199642109–112. [DOI] [PubMed] [Google Scholar]
- 14.Datta P, Frost E, Peeling R.et al Ophthalmia neonatorum in a trachoma endemic area. Sex Transm Dis 1994211–4. [DOI] [PubMed] [Google Scholar]
- 15.Brunham R C, Simonsen J N, Cameron D W.et al The prevalence of Chlamydia trachomatis infection among mothers of children with trachoma. Am J Epidemiol 1990132946–952. [DOI] [PubMed] [Google Scholar]
- 16.Bell T, Stamm W E, Kuo C.et al Delayed appearance of Chlamydia trachomatis infections acquired at birth. Pediatr Infect Dis J 19876928–931. [DOI] [PubMed] [Google Scholar]
- 17.Hammerschlag M R, Cummings C, Robling P M.et al Efficacy of neonatal ocular prophylaxis for the prevention of chlamydial and gonococcal conjunctivitis. N Engl J Med 1989320769–772. [DOI] [PubMed] [Google Scholar]
- 18.Black‐Payne C, Bocchini J A, Cedotal C. Failure of erythromycin ointment for postnatal ocular prophylaxis of chlamydial conjunctivitis. Pediatr Infect Dis J 19898491–498. [DOI] [PubMed] [Google Scholar]
- 19.Laga M, Plummer F A, Piot P.et al Prophylaxis of gonococcal and chlamydial ophthalmia neonatorum: a comparison of silver nitrate and tetracycline. N Engl J Med 1988318653–657. [DOI] [PubMed] [Google Scholar]
- 20.Isenberg S J, Apt L, Del Signore M.et al A double application approach to ophthalmia neonatorum prophylaxis. Br J Ophthalmol 2003871449–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]