Abstract
Aim
The current study was designed to determine whether intravitreal injection of tacrolimus (FK506) modulates the gene expression of neurotrophic factor‐related molecules in the retina from eyes with induced experimental autoimmune uveoretinitis (EAU) in rats.
Methods
Rats were immunised with interphotoreceptor retinoid binding protein peptide (R14) and given intravitreal injection of tacrolimus on day 12 after immunisation. As control, immunised rats received intravitreal injection of vehicle. On day 15 after immunisation, changes in the genetic programme associated with neuroprotection and inflammatory responses in the retinas from both groups were determined by DNA microarray analyses and confirmed by real‐time PCR analyses.
Results
The gene expression of inflammatory responses was markedly reduced in tacrolimus‐treated eyes. Genes for molecules associated with neuroprotection (oestrogen receptor, erythropoietin receptor, gamma‐aminobutyric acid receptor, protein kinase C, glial cell line‐derived neurotrophic factor receptor, fibroblast growth factor and neuropeptide Y receptor) were upregulated in the retinas from tacrolimus‐treated eyes.
Conclusions
Intravitreal injection of tacrolimus modulated the genes related to neuroprotection in the retina during the ongoing process of EAU. This treatment may be useful for the neuroprotection of retina with severe uveitis as well as for immunosuppression in the uveitic eyes.
Tacrolimus (FK506) is a substance isolated and purified from metabolites of a fungus, Streptomyces tsukubaensis.1 Tacrolimus has been used for the prevention of allograft rejection as an immunosuppressive drug.2,3 In addition, tacrolimus has been reported to exert a powerful neuroprotective action in experimental stroke and ischemia.4,5,6,7 Furthermore, a previous study has shown that tacrolimus confers neuroprotection on retinal ganglion cells after optic nerve crush.8
Experimental autoimmune uveoretinitis (EAU) is an inflammatory disease model that shares many clinical and histopathological features with human disease such as Behçet's disease.9,10,11 In animals, EAU can be induced by immunisation with interphoto‐receptor retinoid binding protein (IRBP), an eye‐specific retinal antigen, or by transfer of the antigen‐specific T cells.12,13,14 EAU is a Th1 dominant response to the uveitogenic retinal antigen, and uveitogenic effector T cells display a Th1‐like cytokine profile.15,16
Recently we have demonstrated that intravitreal injection of tacrolimus effectively suppresses ongoing EAU in rats and preserves the retinal structure of eyes with EAU.17 In the current study, we investigated whether intravitreal injection of tacrolimus modulates gene expression of neurotrophic factor‐related molecules in the retinas from eyes with ongoing EAU in rats, using DNA microarrays.
Materials and methods
Animals
Female Lewis rats (6–8 weeks old) were purchased from Japan CLEA (Tokyo, Japan). All rats were treated in accordance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research. A mixture of ketamine HCl and xylazine was used for anesthesia and administered by intraperitoneal injection.
Reagents
Bovine IRBP peptide (R14)14 was prepared using synthesiser (Sawady Technology, Tokyo, Japan). Complete Freund's adjuvant (CFA) was purchased from Difco Labs (Detroit, MI, USA). Killed Bordetella pertussis suspension was purchased from Sigma Chemical (St Louis, MO, USA).
Induction and scoring of EAU
Lewis rats received an injection into one hind footpad of R14 (0.5 μg) in 0.1 ml emulsion in CFA.14 Killed Bordetella pertussis suspension (1×1010 cells) was given intraperitoneally as an additional adjuvant.
Eyes were examined daily after R14 immunisation independently by two blinded observers to assess for the onset of inflammation using a slit‐lamp biomicroscope.18
Intravtireal injection of tacrolimus
Tacrolimus (Prograf, Astellas Pharma Inc, Tokyo, Japan) was dissolved in balanced saline solution (BSS plus; Alcon, Fort Worth, TX, USA) at a concentration of 2 mg/ml. Five μl of tacrolimus solution was injected into the vitreous cavity of rats on day 12 after immunisation using a 30G needle after paracentesis was performed.
DNA microarray hybridisation and analysis
Our recent paper showed that ocular inflammation in the anterior segment was significantly suppressed in the tacrolimus‐treated eyes on day 14 and 16 after immunisation.17 However, both tacrolimus‐ and vehicle‐treated eyes showed decreased inflammation in the anterior segment on day 16 after immunisation.17 Therefore, we collected eyes from both groups on day 15. The tacrolimus‐treated eyes (three eyes) and vehicle‐treated eyes (three eyes) were enucleated on day 15 after immunisation. Total RNA was extracted from whole retina of enucleated eyes using an Isogen RNA isolation kit (Nippon Gene, Tokyo, Japan). The analysis of RNA quality showed that the 260 : 280 nm absorbance ratio of RNA samples used in this experiment ranged consistently from 1.8 to 2.0. The integrity and concentration of total RNA were measured using a bioanalysis unit (Agilent 2100 Bioanalyser; Agilent Technologies, Palo Alto, CA, USA). Fluorescence‐labelled antisense RNA was synthesised by direct incorporation of Cy3‐UTP or Cy5‐UTP (GE Healthcare Bio‐Science Corp., Piscataway, NJ, USA) using 2 μg of each RNA sample and an RNA Transcript SureLABEL Core Kit (TaKaRa BIO Inc., Tokyo, Japan). The labelled antisense RNAs were hybridised simultaneously to the microarray chip (FilgenArray Rat 27k, Filgen, Inc., Nagoya, Japan). Array hybridisation was performed according to the manufacturer's instructions (Filgen, Inc.). The fluorescence images of hybridised microarrays were obtained with a GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA, USA). The Array‐Pro Analyzer Ver4.5 (Media Cybernetics, Inc., Silver Spring, MD, USA) was used to determine the signal intensity of each spot and its local background. The net intensity was calculated by subtracting the mean intensity of all pixels within the local background area from the mean intensity of all pixels within the spot areas. The biases in net intensity were normalised by locally weighted linear regression analysis. Analysed data were selected using a MicroArray Data Analysis Tool (Filgen, Inc.).
Real‐time PCR
First strand cDNAs were synthesised using TaqMan(R) One‐step RT‐PCR Master Mix Reagents (Applied Biosystems, Foster City, CA, USA) from total RNA. Levels of oestrogen receptor 2 beta and erythropoietin receptor were determined over the time course by real‐time PCR (TaqMan chemistry with the ABI Prism 7900HT sequence Detection System; Applied Biosystems). TaqManGeneExpressionAssays™ TaqMan™ probes and primer pairs of erythropoietin (assay ID: Rn00690244_g1), oestrogen receptor 2 beta (assay ID: Rn00688791_m1) and GAPDH (assay ID: Rn01775763_g1) were obtained from Applied Biosystems.
For relative quantification in real‐time PCR experiments, we used the comparative Ct methods (Applied Biosystems). Samples were assayed using thermal cycler conditions consisting of 10 min at 48°C and 10 min at 95°C, followed by 60 cycles at 95°C for 15 s and 60°C for 1 min.
Results
Comparison of gene expression profiles in tacrolimus‐treated eyes and vehicle‐treated eyes by DNA microarray analysis
Slit‐lamp examination demonstrated that mean clinical EAU scores on day 12 after immunisation were 3.3 in tacrolimus‐treated rats and 3.5 in vehicle treated rats. On day 14, mean clinical EAU scores were 0.5 in tacrolimus‐treated rats, and 5.7 in vehicle‐treated rats, indicating that intravitreal injection of tacrolimus suppressed ongoing EAU. On day 15, eyes were enucleated from both groups and retinas were collected for RNA extraction. The total RNA was applied for DNA microarray analysis.
The signal ratio of each of the 28 800 genes was calculated. Genes with a twofold ratio increase were defined arbitrarily as upregulated in tacrolimus‐treated eyes, whereas those with a ratio decreased by one‐half or more were defined as downregulated. Using these criteria, 1828 genes were found to be upregulated, and 1594 genes were found to be downregulated, in tacrolimus‐ treated eyes compared with vehicle‐treated eyes.
Summaries of genes differentially expressed between two groups (tacrolimus‐treated eyes/vehicle‐treated eyes) are shown in Tables 1 and 2. The differentially expressed genes are arranged according to two functional categories based on known functions of these genes: (1) inflammation‐related genes and (2) neuroprotection related genes. As demonstrated in Table 1, the gene expression of interleukin (IL)‐1 receptor type 1, inducible nitric oxide synthase 2, Il‐6, C‐C ligand 5 (Ccl5), CD3 antigen, CC chemokine receptor 7 (Ccr7), CXC ligand 9 (Cxcl9) (also known as monokine induced by interferon gamma (Mig)) and IL‐2 receptor gamma was downregulated in retinas from tacrolimus‐treated eyes.
Table 1 Differential expression of inflammation‐related genes in retinas derived from uveitic eyes receiving intravitreal injection of tacrolimus.
| Common name | GenBank Accession No. | Ratio |
|---|---|---|
| IL‐1 receptor type 1 | M95578 | 0.01 |
| Nitric oxide synthase 2, inducible | U03699 | 0.21 |
| Il‐6 | M26744 | 0.23 |
| C‐C ligand 5 (Ccl5) | – | 0.31 |
| CD3 antigen, gamma polypeptide | BF560704 | 0.32 |
| Ccr7 | BC089762 | 0.35 |
| CXC ligand 9 (Cxcl9, also known as Mig) | BC087594 | 0.36 |
| IL‐2 receptor gamma | BC079343 | 0.49 |
IL, interleukin; Ccr, CC chemokine receptor; Mig, monokine induced by interferon gamma.
Table 2 Differential expression of neurotrophic factor‐related genes in retinas derived from uveitic eyes receiving intravitreal injection of tacrolimus.
| Common name | GenBank Accession No. | Ratio |
|---|---|---|
| Oestrogen receptor 2 beta | U57439 | 4.51 |
| Erythropoietin receptor | BC089810 | 3.31 |
| Similar to Bcl‐x, short | – | 2.78 |
| GABA A receptor, gamma 1 | X57514 | 2.72 |
| Protein kinase C, alpha | – | 2.28 |
| GDNF receptor family receptor alpha 4 | AJ294476 | 2.25 |
| Fibroblast growth factor 8 | – | 2.17 |
| Neuropeptide Y receptor Y1 | BC089981 | 2.15 |
| Protein kinase C, beta 1 | X04440 | 2.08 |
GDNF, glial cell line derived neurotrophic factor receptor.
On the other hand, as shown in Table 2, the expression of neutroprotection‐related genes such as oestrogen receptor 2 beta, erythropoietin receptor, GABA receptor, protein kinase C, glial cell line‐derived neurotrophic factor receptor, fibroblast growth factor and neuropeptide Y receptor was elevated in the retinas from tacrolimus‐treated eyes.19,20,21,22,23,24,25,26,27,28,29,30
Confirmation of differentially expressed genes
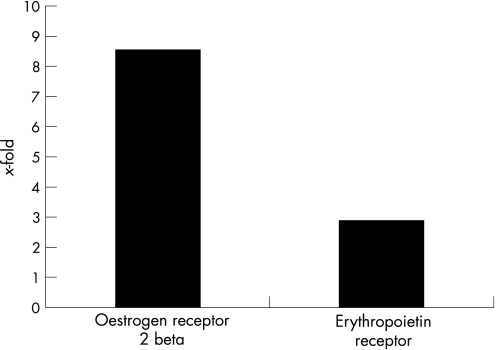
To confirm that observed differences in DNA microarray analysis correlated with differences in steady levels of the corresponding mRNAs, the expression patterns of selected genes were determined by real time PCR. Levels of selected genes relative to that of a housekeeping gene (glyceraldehyde‐3‐phosphate dehydrogenase [GADPH]) were compared between tacrolimus‐treated eyes and vehicle‐treated eyes. This method confirmed the differential expression of each of the selected genes in the expected manner (Fig. 1). mRNA expression of oestrogen receptor 2 beta and erythropoietin receptor was elevated in retinas from tacrolimus‐treated eyes.
Figure 1 Real‐time PCR assay of relative mRNA levels of the oestrogen receptor 2 beta and erythropoietin receptor in retina from tacrolimus‐treated eyes and vehicle‐treated eyes (GAPDH served as the reference gene; relative expression of each gene was determined from the Ct): changes in expression (x‐fold) of selected upregulated genes in retina from tacrolimus‐treated eyes compared with those from vehicle‐treated eyes.
Discussion
We recently demonstrated that intravitreal injection of tacrolimus is capable of suppressing the ongoing process of autoimmune uveoretinitis in the eye and can preserve the retinal structure of the uveitic eye.17 Tacrolimus has been shown to exert profound neuroprotective and neuroregenerative effects in vivo and in vitro.4,5,7,31 However, the underlying molecular pathways of neuroprotection are not fully understood. We used DNA microarray technology to define genes that are related to neuroprotection in the retinas from tacrolimus treated eyes with EAU. We obtained evidences that intravitreal injection of tacrolimus upregulated the gene expression of neuroprotection‐related molecules as well as decreased the expression of inflammatory responses related genes. These data support the notion that increased expression of neuroprotection‐related genes by intravitreal injection of tacrolimus may play a potential role in retinal protection of the eyes with ongoing ocular inflammation, as well as in immune regulation.
Microarray analysis showed that gene expression of oestrogen receptor 2 was upregulated in the retinas derived from tacrolimus‐treated eyes. In addition, we also observed that protein kinase C gene expression was increased in tacrolimus‐treated eyes. Recent reports have demonstrated a neuroprotective effect of oestrogen.19,20,21 Furthermore, Cordey et al. have shown that oestrogen‐induced neuroprotection of neurons depends on activation of protein kinase C.26,27 These reports, together with the results of the microarray study, suggest that upregulation of oestrogen receptor 2 and protein kinase C genes by intravitreal injection of tacrolimus may be a important factor involved in the preservation of sensory retina with ongoing EAU.
Erythropoietin receptor was induced in the tacrolimus‐treated retina. So far, several experimental studies have shown that both erythropoietin and the erythropoietin receptor are functionally expressed in the nervous system and that this cytokine exerts a remarkable neuroprotection.22,23,24 In the field of ophthalmology, it has been reported that erythropoietin has a potential role as therapeutic molecule against apoptotic neuronal cell death in the context of glaucoma or neurodegenerative diseases.32,33 Taken together, the upregulation of erythropoietin receptor induced by tacrolimus delivered into vitreous cavity may play a part in the neuroprotection of sensory retina in the uveitic eyes.
In conclusion, the results of this DNA microarray experiment suggest that upregulation of neurotrophic factor‐related gene expression and downregulation of inflammation related genes may be important mechanisms by which intravitreal injection of tacrolimus not only prevent ocular inflammation but also facilitates preservation of retinal architecture in the eyes with uveoretinitis.
Acknowledgements
The authors are indebted to JP Barron of the International Medical Communications Center of Tokyo Medical University for his review of this manuscript.
Abbreviations
EAU - experimental autoimmune uveoretinitis
IL - interleukin
IRBP - interphotoreceptor retinoid binding protein
Footnotes
Funding: This work was supported by Grant‐in‐Aid 17791258 for Scientific Research from the Japan Society for the Promotion of Science.
Competing interests: None.
References
- 1.Kino T, Hatanaka H, Hashimoto M.et al FK‐506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico‐chemical and biological characteristics. J Antibiot (Tokyo) 1987401249–1255. [DOI] [PubMed] [Google Scholar]
- 2.Wong W, Venetz J P, Tolkoff‐Rubin N.et al 2005 immunosuppressive strategies in kidney transplantation: which role for the calcineurin inhibitors? Transplantation 200580289–296. [DOI] [PubMed] [Google Scholar]
- 3.Wente M N, Sauer P, Mehrabi A.et al Review of the clinical experience with a modified release form of tacrolimus [FK506E (MR4)] in transplantation. Clin Transplant. 2006;20(Suppl 17)80–84. [DOI] [PubMed]
- 4.Butcher S P, Henshall D C, Teramura Y.et al Neuroprotective actions of FK506 in experimental stroke: in vivo evidence against an antiexcitotoxic mechanism. J Neurosci 1997176939–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuichi Y, Noto T, Li J Y.et al Multiple modes of action of tacrolimus (FK506) for neuroprotective action on ischemic damage after transient focal cerebral ischemia in rats. Brain Res 20041014120–130. [DOI] [PubMed] [Google Scholar]
- 6.Zawadzka M, Kaminska B. A novel mechanism of FK506‐mediated neuroprotection: downregulation of cytokine expression in glial cells. Glia 20054936–51. [DOI] [PubMed] [Google Scholar]
- 7.Labrande C, Velly L, Canolle B.et al Neuroprotective effects of tacrolimus (FK506) in a model of ischemic cortical cell cultures: role of glutamate uptake and FK506 binding protein 12 kDa. Neuroscience 2006137231–239. [DOI] [PubMed] [Google Scholar]
- 8.Freeman E E, Grosskreutz C L. The effects of FK506 on retinal ganglion cells after optic nerve crush. Invest Ophthalmol Vis Sci 2000411111–1115. [PubMed] [Google Scholar]
- 9.Caspi R R, Roberge F G, Chan C C.et al A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol 19881401490–1495. [PubMed] [Google Scholar]
- 10.Chan C C, Caspi R R, Ni M.et al Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun 19903247–255. [DOI] [PubMed] [Google Scholar]
- 11.Forrester J V, Liversidge J, Dua H S.et al Experimental autoimmune uveoretinitis: a model system for immunointervention: a review. Curr Eye Res 199211(Suppl)33–40. [DOI] [PubMed] [Google Scholar]
- 12.Gery I, Wiggert B, Redmond T M.et al Uveoretinitis and pinealitis induced by immunization with interphotoreceptor retinoid‐binding protein. Invest Ophthalmol Vis Sci 1986271296–1300. [PubMed] [Google Scholar]
- 13.Caspi R R, Roberge F G, McAllister C G.et al T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol 1986136928–933. [PubMed] [Google Scholar]
- 14.Sanui H, Redmond T M, Kotake S.et al Identification of an immunodominant and highly immunopathogenic determinant in the retinal interphotoreceptor retinoid‐binding protein (IRBP). J Exp Med 19891691947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi R R, Silver P B, Chan C C.et al Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol 19961572668–2675. [PubMed] [Google Scholar]
- 16.Caspi R R, Sun B, Agarwal R K.et al T cell mechanisms in experimental autoimmune uveoretinitis: susceptibility is a function of the cytokine response profile. Eye. 1997;11(Pt 2)209–212. [DOI] [PubMed]
- 17.Oh‐i K, Keino H, Goto H.et al Intravitreal injection of Tacrolimus (FK506) suppresses ongoing experimental autoimmune uveoretinitis in Rats. Br J Ophthalmol 200691237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada A A, Keino H, Fukai T.et al Effect of type I interferon on experimental autoimmune uveoretinitis in rats. Ocul Immunol Inflamm 19986215–226. [DOI] [PubMed] [Google Scholar]
- 19.Littleton‐Kearney M T, Ostrowski N L, Cox D A.et al Selective estrogen receptor modulators: tissue actions and potential for CNS protection. CNS Drug Rev 20028309–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin R, Guerra B, Alonso R.et al Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res 20052287–301. [DOI] [PubMed] [Google Scholar]
- 21.Cordey M, Pike C J. Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res 20051045217–223. [DOI] [PubMed] [Google Scholar]
- 22.Aydin A, Genc K, Akhisaroglu M.et al Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic–ischemic brain injury. Brain Dev 200325494–498. [DOI] [PubMed] [Google Scholar]
- 23.Genc S, Koroglu T F, Genc K. Erythropoietin and the nervous system. Brain Res 2004100019–31. [DOI] [PubMed] [Google Scholar]
- 24.Bartesaghi S, Marinovich M, Corsini E.et al Erythropoietin: a novel neuroprotective cytokine. Neurotoxicology 200526923–928. [DOI] [PubMed] [Google Scholar]
- 25.Paula‐Lima A C, De Felice F G, Brito‐Moreira J.et al Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta‐amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005491140–1148. [DOI] [PubMed] [Google Scholar]
- 26.Cordey M, Gundimeda U, Gopalakrishna R.et al Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem 2003841340–1348. [DOI] [PubMed] [Google Scholar]
- 27.Cordey M, Pike C J. Conventional protein kinase C isoforms mediate neuroprotection induced by phorbol ester and estrogen. J Neurochem 200696204–217. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Wu J P, Tzeng S F. Neuroprotection of glial cell line‐derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res 200269397–405. [DOI] [PubMed] [Google Scholar]
- 29.Mark R J, Fuson K S, Keane‐Lazar K.et al Fibroblast growth factor‐8 protects cultured rat hippocampal neurons from oxidative insult. Brain Res 199983088–93. [DOI] [PubMed] [Google Scholar]
- 30.Wang S J. Activation of neuropeptide Y Y1 receptors inhibits glutamate release through reduction of voltage‐dependent Ca2+ entry in the rat cerebral cortex nerve terminals: suppression of this inhibitory effect by the protein kinase C‐dependent facilitatory pathway. Neuroscience 2005134987–1000. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki Y, Ichikawa Y, Igarashi O.et al Neuroprotective actions of FK506 and cyclosporin A on motor neuron survival following neonatal axotomy. Neurol Res 200224573–576. [DOI] [PubMed] [Google Scholar]
- 32.Weishaupt J H, Rohde G, Polking E.et al Effect of erythropoietin axotomy‐induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 2004451514–1522. [DOI] [PubMed] [Google Scholar]
- 33.Kilic U, Kilic E, Soliz J.et al Erythropoietin protects from axotomy‐induced degeneration of retinal ganglion cells by activating ERK‐1/‐2. FASEB J 200519249–251. [DOI] [PubMed] [Google Scholar]