Abstract
Aim
The risk of regression after photorefractive keratectomy (PRK) and the tendency to develop keratectasia after laser‐assisted in situ keratomileusis (LASIK) procedure is higher in women than men. Currently, interest is focused on the influence of oestrogen on corneal stability after corneal refractive surgery. The aim of this experimental study was to investigate the change in biomechanical properties of the cornea induced by oestrogen.
Methods
The influence of oestrogen was investigated in 12 fresh porcine corneas incubated in culture medium with 10 μmol/l β‐oestradiol for 7 days. A group of 12 porcine corneas incubated in culture medium without oestradiol for the same time served as a control group. Strips of cornea were cut and the stress–strain was measured in a biomaterial tester. The Young's modulus was calculated.
Results
During incubation the thickness of the cornea changed in the control group by only 6.4% and in the oestradiol group by 12%. However, the difference in the biomechanical stress values at 10% strain was significantly larger. In the control group the stress value was 120.18±28.93 kPa and in the oestradiol group 76.87±34.63 kPa (p = 0.002), representing a reduction of the corneal stiffness by 36% due to the oestradiol treatment.
Conclusion
Oestrogen is a modulating factor of the biomechanical properties of the cornea that is not explainable only by an increased swelling. The significance of the hormone status of patients and its influence on the biomechanical stability of the cornea, a determining factor after refractive surgery, have been underestimated and may contribute to the development of keratectasia.
Changes of the thickness of the cornea due to fluctuations of oestrogen level in blood are known from measurements during the menstrual cycle1,2 and also during pregnancy.3 Corneal curvature also changes during pregnancy.4 Women taking contraceptives5 report problems with hard contact lenses and pregnant women frequently complain of contact lens intolerance.6 Currently, much interest is focused on research into the influence of oestrogen on the cornea in relation to corneal stability after corneal refractive surgery, as seen in studies concerning regression after photorefractive keratectomy (PRK) and after laser‐assisted in situ keratomileusis (LASIK).7 The risk of regression after PRK is 13.5 times higher in women taking oral contraceptives.8 Women taking hormone replacement therapy have a higher risk for post‐LASIK refractive regression.9 Women have a ninefold higher risk for developing keratectasia after LASIK than men.10
Since a regression after PRK or the development of keratectasia after excimer laser ablation is related to a reduction of the stiffness of the cornea, it has been postulated that oestrogen possesses a structure‐modulating effect on the cornea leading to an alteration of corneal biomechanics. To date, however, no studies have been published regarding the influence of oestrogen on the biomechanical behaviour of the cornea.
Therefore, the aim of this experimental study was to investigate the change of the biomechanical properties of the cornea induced by oestrogen.
Methods
The influence of oestrogen was investigated in 12 fresh porcine corneas. The corneo‐scleral buttons were prepared from porcine eyes enucleated from 4–5‐month‐old pigs of both sexes of the local abattoir within 5 h post mortem. β‐Oestradiol (E2257) (Sigma‐Aldrich, Munich, Germany) was dissolved in 100% ethanol to a final stock concentration of 20 μg/ml, which was further diluted to achieve the desired concentration of steroid. The diluted oestrogen solution was added to the corneas in a final working concentration 10 μmol/l. The oestrogen concentration of 10 μmol/l was chosen from other in vivo studies that investigated the influence of oestrogen on lens epithelial cells.11,12 During pregnancy the oestrogen concentration reaches 100 nmol/l and the pharmacological concentration may reach values up to 10 μmol/l.12
The corneo‐scleral buttons were excised and organ cultivated in medium MEM (Biochrom AD, Berlin, Germany), containing penicillin (100 U/ml)/streptomycin (100 μg/ml), supplemented with 2% fetal calf serum (Invitrogen, Karlsruhe, Germany) for 7 days at 37°C in an incubator under 5% CO2 atmosphere. Cell culture media for all samples contained phenol red as pH indicator that allowed visual observation of pH change in the media due to cell metabolism or environment factors. The culture medium in each group was enriched with 4% Dextran T500 (Steromed Biochrom, Berlin, Germany) to avoid swelling of the corneas. The incubation time of 7 days was chosen according to the study of the influence of oestrogen on the skin in mice.13
As a control group 12 porcine corneas were incubated in culture medium without β‐oestradiol for 7 days. Strips of 10×5 mm were cut from corneas from superior to inferior and the stress–strain relationship was measured with a microcomputer‐controlled biomaterial tester (Minimat; Rheometric Scientific GmbH, Benshaim, Germany) at room temperature (22°C). The corneal strips were clamped and a prestress of 5×103 N/m2 was applied for 3 min. After that, the strain was increased linearly with a velocity of 2 mm/min and the stress was detected. The stress–strain curves were evaluated and the Young's modulus E determined. Measurement of the central corneal thickness using an ultrasonic pachymeter (Pach‐Pen XL; Mentor, Norwell, MA, USA) was necessary for the calculation of the stress values. The thickness of the corneas was measured before incubation (original thickness) and after 7 days incubation (thickness after incubation). For statistical calculations the Student's t test was used.
Results
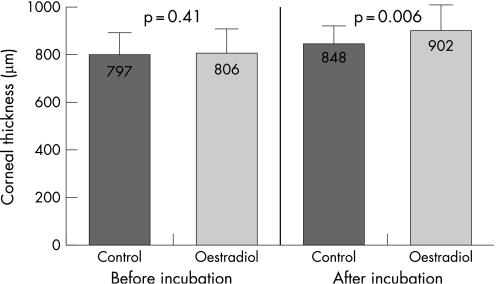
The corneal thickness in the oestrogen group increased during the 7 days of incubation in culture medium containing 10 μmol/l β‐oestradiol by 96 μm (12%), from 806±72 μm to 902±98 μm, while the increase of thickness in the control group incubated in culture medium without β‐oestradiol was only 51 μm (6.4%), from 797±56 μm to 848±54 μm (Fig. 1). This different increase in thickness between the two groups is statistically significant (p = 0.006).
Figure 1 Corneal thickness before incubation and after incubation for the control and oestrogen groups.
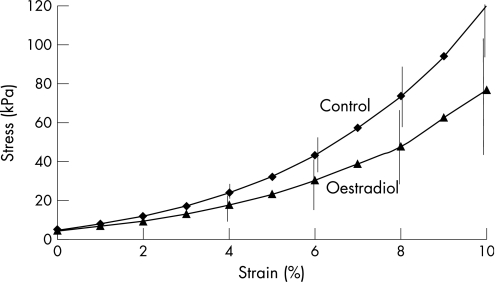
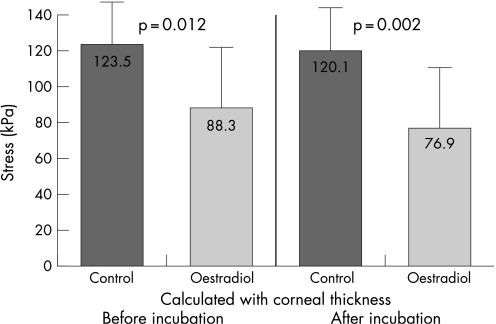
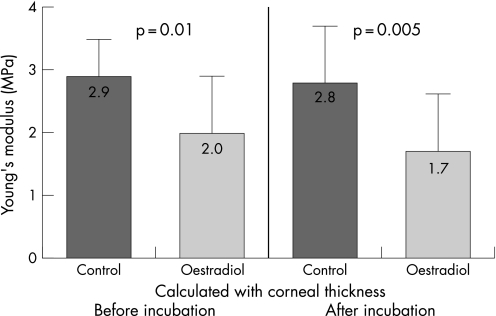
Fig. 2 illustrates the different stress–strain behaviour of the control and oestradiol‐treated corneas. For statistical evaluation of the biomechanics, the stress values were compared at 10% strain. The mean values were in the control group calculated with original thickness 123.54±30.86 kPa (E = 2.9±0.7 MPa), in the oestrogen group calculated with original thickness 88.33±34.38 kPa (E = 2.0±0.8 MPa), in the control group calculated with corneal thickness after incubation 120.18±28.93 kPa (E = 2.8±0.8 MPa), and in the oestrogen group calculated with corneal thickness after incubation 76.87±34.63 kPa (E = 1.7±0.8 MPa). The values of stress and of Young's modulus of the two groups are shown in Figs 3 and 4.
Figure 2 Stress–strain behaviour of control and oestradiol‐treated corneas.
Figure 3 Mean stress values at 10% strain for control and oestrogen groups calculated with corneal thickness before and after 7 days' incubation.
Figure 4 Young's modulus of control and oestogen groups.
Taking into consideration the change of corneal thickness during incubation, the stress in the oestrogen group was significantly diminished by 36% (p = 0.002) in comparison with the control group. Comparison of the stress of the oestrogen group calculated with corneal thickness before and after incubation shows a reduction of 12.9%, which is not statistically significant (p = 0.371). Thus, the change of thickness alone cannot explain this high biomechanical effect. The main effect of the reduction of the stiffness may be seen from the comparison of the control group and the oestrogen group assuming the original thickness before incubation. In this case the stress is reduced by 28.5% and the Young's modulus is reduced by 30% from E = 2.9±0.7 MPa to E = 2.0±0.8 MPa.
Discussion
This experimental investigation shows a significant influence of oestrogen on the corneal thickness and also on the biomechanics of the cornea. It is the first study showing a direct influence of oestrogen on the biomechanical behaviour of the cornea. Previous reports showed an increase of the corneal thickness1,2,3,14 resulting from an elevated hydration.15 There was a direct linear relationship between hydration and the corneal thickness.16 All changes in structure of the cornea, such as swelling, influence the mechanical and optical properties and affect the function of the eye.17 The effect of hydration of the cornea on refraction after radial keratotomy was shown18 and theoretically explained.19 The biomechanical stress σ is calculated by the force F divided by the cross‐section area A. Since the cornea swells only in one direction, the swelling is reflected in the corneal thickness. If oestrogen produces only a simple swelling process we would expect a decrease of the stress value of about 6%. However, we found a reduction of the stress of 36%, which means that oestrogen induces further, more complex and deeper biomechanical changes than swelling.
Oestrogen has a stiffness‐reducing effect on the cornea that does not play an important role under normal conditions but can be clearly seen in a biomechanically weakened cornea, such as after PRK8 or LASIK9,10, and also in cases of keratoconus after puberty20,21,22,23,24 or after abortion due to the increase of oestrogen activity.25
This stiffness‐changing effect of oestrogen may be explained at the molecular level. The primary level of action of oestrogen is the regulation of gene expression. Oestrogen has been identified in the tear film and in aqueous humor with a concentration nearly one‐half of the plasma concentration.26,27 It is a steroid hormone acting on specific receptors. Due to its low molecular mass (272.4), oestrogen diffuses into the cells and connects to specific nucleus‐receptors. Keratocytes of the human cornea of both sexes contain such oestrogen receptors (OR‐α and OR‐β), where oestrogen initiates the biochemical changes.28 The oestrogen–receptor complex in the cell nucleus then acts as a hormone‐induced transcription factor and regulates immediately the gene transcription and thus the intracellular protein synthesis. The increased (or decreased) synthesis of mRNA leads to changed concentrations of proteins in the extracellular matrix as well. Thus oestrogen has a profound influence on composition of cornea. For that reason the cornea is an oestrogen‐sensitive tissue, and therefore the biological function of the keratocytes will be influenced by the direct interaction with the hormones. The synthesis of biomolecules is responsible for biomechanical stability and is affected by this cascade.
The stiffness of the cornea is determined by the extracellular matrix consisting mainly of collagens, proteoglycans and glycosaminoglycans.
Oestradiol stimulates the production of matrix metalloproteinases29, and the production or activation of collagenolytic enzymes may be responsible for the weakening of the collagen network.30 Oestrogen increases the prostaglandin release and thereby activates collagenases,31 acting like a chain breaker.32 This cause a degradation of type I collagen.33 For the biomechanics of the cornea this means an increased distensibility, but a reduced stiffness.
The viscoelastic behaviour of the cornea is determined also by the proteoglycans and not only by the collagens. Proteoglycans play an important role in the arrangement (distance) of the collagen fibres and in the cohesive force between the lamellae. They act as an “interfibrillar or interlamellar glue”. It is possible that this cohesion between collagen fibrils and the non‐collagenous matrix may be reduced by oestrogen, which facilitates the slippage of collagen within the stroma.34
Glycosaminoglycans, as part of the proteoglycans, are responsible for water‐binding capacity and for the flexibility of the tissues. Oestrogen stimulates the synthesis of glycosaminoglycans combined with an enriching of water and an increase of corneal thickness. This leads to a weakening of the collagen network.35,36,37 The water enrichment seems to be very important in separating the collagen fibres. The oestrogen‐induced increasing water‐holding capacity of the glycosaminoglycans enables it to act as an osmotic expander, which leads to an increase of the interfibrillar space17 and mechanical expansion of the crosslinks between the collagen (lamellae or fibrils). Since oestrogen receptors are found also in endothelial cells of the cornea it may be presumed that oestrogen influences the pump function of the endothelium. However, similar changes are observed in the skin, vessel wall or cartilage where endothelium does not play such a function.
From studies on the skin it is known that an increase in the thickness of the skin, combined with an accumulation of water, is the result of the oestrogen‐induced synthesis of hyaluronic acid.38 The water content in the skin can be nearly doubled13 and the thickness of the skin increases by 7–15% after hormone replacement therapy. In contrast to the cornea, the influence of oestrogen on the biomechanical properties of the skin has already been investigated.39
The association between oestrogen and biomechanical modifications has been reported for other biological tissues. Similar reduced biomechanical stiffness was also found at the anterior cruciate ligament in rabbits at higher oestrogen levels.40
Not only does an elevated oestrogen level results in biomechanical changes, but also a decreased level. The level of glycosaminoglycans is significantly reduced in cartilage in cases of oestrogen deficiency and this is followed by an increase of cartilage stiffness.41 From previous studies it is well‐known that a degradation of glycosaminoglycans leads to an elevated biomechanical stiffness of tissues.42,43 In addition, an increase of stiffness with lower compliance of the blood vessels was observed after oestrogen reduction.44,45
Previous investigations have shown the relationship between the degree of hydration and the biomechanical properties (especially the viscoelastic behaviour) of the cornea,35,46 but a connection with an oestrogen‐induced changes has not yet been reported. Soergel found a reduction of shearing strength with increasing hydration.35 Hjortdal46 revealed in his experimental investigation a significantly increased distensibility of the cornea at higher hydration level.
An increased oestrogen level may be observed during puberty, during the menstrual cycle, during pregnancy, in hormone replacement therapy and in some cases of abortion. It is conceivable that keratoconus may be triggered by oestrogen possibly partly through a genetic predispostion for a weak cornea after puberty.
In a normal pregnancy the oestrogen level increases about by a factor of 20, but the biomechanical effect of oestrogen is widely compensated by the hormone progesterone. Progesterone inhibits prostaglandin production whereas oestrogen increases prostaglandin synthesis. Prostaglandins lead to an increase of collagenases. Progesterone does not only suppress the production of collagenases,47 but it also inhibits activated collagenases by proteinase inhibitor (tissue inhibitor of metalloproteinases, TIMP).48 Progesterone insufficiency (especially a low level of progesterone) may lead to abortion, which combined with an elevated activity of oestrogen may also influence the biomechanics of the cornea25 and cause corneal ectasia. Hormone replacement therapy may also influence the biomechanical stability of the cornea and should be considered in cornea‐weakening procedures.
In summary, oestrogen is a modulating factor of the biomechanical properties of the cornea that is not only explainable by an increased swelling. Therefore the influence of the hormone status on the biomechanical stability of the cornea, and even more so on the results of corneal refractive procedures leading to the development of keratectasia, should not be underestimated.
Abbreviations
LASIK - laser‐assisted in situ keratomileusis
PRK - photorefractive keratectomy
References
- 1.Kielly P M, Carney L G, Smith G. Menstrual cycle variations of corneal topography and thickness. Am J Optom Physiol Opt 198360822–829. [DOI] [PubMed] [Google Scholar]
- 2.Giuffre G, Di Rosa L, Fiorino F.et al Variations in central corneal thickness during the menstrual cycle in women. Cornea 200726144–146. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb R N, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol 1988105258–260. [DOI] [PubMed] [Google Scholar]
- 4.Park S B, Lindahl K J, Temnycky G O.et al The effect of pregnancy on corneal curvature. CLAO J 199218256–259. [PubMed] [Google Scholar]
- 5.Soni P S. Effects of oral contraceptive steroids on the thickness of human cornea. Am J Optom Physiol Optics 198057825–834. [DOI] [PubMed] [Google Scholar]
- 6.Imafidon C O, Imafidon J E. Contact lens wear in pregnancy. J Br Contact Lens Assoc 19911475–78. [Google Scholar]
- 7.McCarty C A, NG I. Waldron B.et al Relation of hormone and menopausal status to outcomes following excimer laser photorefractive keratectomy in women. Melbourne Excimer Laser Group. Aust N Z J Ophthalmol 199624215–222. [DOI] [PubMed] [Google Scholar]
- 8.Corbett M C, O'Brart D P, Warburton F G.et al Biologic and environmental risk factors for regression after photorefractive keratectomy. Ophthalmology 19961031381–1391. [DOI] [PubMed] [Google Scholar]
- 9.O'Doherty M A, O'Doherty J V, O\Keefe M. Outcome of LASIK for myopia in women on hormone replacement therapy. J Refract Surg 200622350–353. [DOI] [PubMed] [Google Scholar]
- 10.Randleman J B, Russell B, Ward M A.et al Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 2003110267–275. [DOI] [PubMed] [Google Scholar]
- 11.Flynn J M, Cammarata P R. Estradiol attenuates mitochondrial depolarization in polyol‐stressed lens epithelial cells. Mol Vis 200612271–282. [PubMed] [Google Scholar]
- 12.Wang X, Simpkins J W, Dykens J A.et al Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability. Invest Ophthalmol Vis Sci 2003442067–2075. [DOI] [PubMed] [Google Scholar]
- 13.Grosman N, Hvidberg E, Schou J. The effect of oestrogenic treatment on the acid mucopolysaccharide pattern in skin of mice. Acta Pharmacol Toxicol (Copenh) 197130458–464. [DOI] [PubMed] [Google Scholar]
- 14.Affinito P, Di Spiezio S A, Di Carlo C.et al Effects of hormone replacement therapy on ocular function in postmenopause. Menopause 200310482–487. [DOI] [PubMed] [Google Scholar]
- 15.Leach N E, Wallis N E, Lothringer L L.et al Corneal hydration changes during the normal menstrual cycle – preliminary study. J Reprod Med 19716201–204. [PubMed] [Google Scholar]
- 16.Hedbys B O, Mishima S. The thickness–hydration relationship of the cornea. Exp Eye Res 19665221–228. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Meek K M. Swelling studies on the cornea and sclera: the effect of pH and ionic strength. Biophys J 1999771655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloney R K. Effect of corneal hydration and intraocular pressure on keratometric power after experimental radial keratotomy. Ophthalmology 199097927–933. [DOI] [PubMed] [Google Scholar]
- 19.Roy P, Petroll W M, McKinney A E.et al Computational models of the effects of hydration on corneal biomechanics and the results of radial keratotomy. J Biomech Eng 1996118255–258. [DOI] [PubMed] [Google Scholar]
- 20.Bechrakis N, Blom M L, Stark W J.et al Recurrent keratoconus. Cornea 19941373–77. [DOI] [PubMed] [Google Scholar]
- 21.Kruse K.Pädiatrische Endokrinologie, Stuttgart, Enke 1993163–165.
- 22.Alonso L C, Rosenfield R L. Oestrogens and puberty. Best Pract Res Clin Endocrinol Metabol 20021613–30. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Attanasio A, Raaf A. Plasma estrogen and androgen concentrations in children during adolescence. J Clin Endocrinol Metab 197540636–643. [DOI] [PubMed] [Google Scholar]
- 24.Olivares Jimenez J L, Guerrero Jurado J C, Bermudez Rodriguez F J.et al Keratoconus: age of onset and natural history. Optom Vis Sci 199774147–151. [DOI] [PubMed] [Google Scholar]
- 25.Di Renzo G C, Mattei A, Gojnic M.et al Progesterone and pregnancy. Curr Opin Obstet Gynecol 200517598–600. [DOI] [PubMed] [Google Scholar]
- 26.Ogueta S B, Schwartz S D, Yamashita C K.et al Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci 1999401906–1911. [PubMed] [Google Scholar]
- 27.Wickham L A, Gao J, Toda I.et al Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand 200078146–153. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Kinoshita Y, Tachibana M.et al Expression of sex steroid hormone receptors in human cornea. Curr Eye Res 20012228–33. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki T, Sullivan D A. Estrogen stimulation of pro‐inflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea 2005241004–1009. [DOI] [PubMed] [Google Scholar]
- 30.Harries E D, Jr, Krane S M. Collagenases. N Engl J Med 197426652–661. [DOI] [PubMed] [Google Scholar]
- 31.Uchihori Y, Inokuchi N, Ikeda T.et al Vitreous estrogen levels in subjects with idiopathic macular hole. Invest Ophthalmol Vis Sci. 2000;41:Abstr 1791
- 32.Niki E, Nakano M. Estrogen as antioxidants. Methods Enzymol 1990186330–333. [DOI] [PubMed] [Google Scholar]
- 33.Rajabi M, Solomon S, Poole A R. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology 1991128863–871. [DOI] [PubMed] [Google Scholar]
- 34.Meek K M, Tuft S J, Huang Y.et al Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci 2005461948–1956. [DOI] [PubMed] [Google Scholar]
- 35.Soergel F, Jean B, Benedikt J.et al Dynamic mechanical spectroscopy of the cornea for measurement of its viscoelastic properties in vitro. Ger J Ophthalmol 19954151–156. [PubMed] [Google Scholar]
- 36.Mogilner I G, Ruderman G, Grigera J R. Collagen stability, hydration and native state. J Mol Graph Model 200221209–213. [DOI] [PubMed] [Google Scholar]
- 37.Chan R W, Tayama N. Biomechanical effects of hydration in vocal fold tissues. Otolaryngol Head Neck Surg 2002126528–537. [DOI] [PubMed] [Google Scholar]
- 38.Verdier‐Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol 20061583–94. [DOI] [PubMed] [Google Scholar]
- 39.Pierard G E, Letawe C, Dowlati A.et al Effect of hormone replacement therapy for menopause on the mechanical properties of skin. J Am Geriatr Soc 199543662–665. [DOI] [PubMed] [Google Scholar]
- 40.Komatsuda T, Sugita T, Sano H.et al Does estrogen alter the mechanical properties of the anterior cruciate ligament? An experimental study in rabbits. Acta Ortho 200677973–980. [DOI] [PubMed] [Google Scholar]
- 41.Claassen H, Cellarius C, Scholz‐Ahrens K E.et al Extracellular matrix changes in knee joint cartilage following bone‐active drug treatment. Cell Tissue Res 2006324279–289. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt M B, Mow V C, Chun L E.et al Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res 19908353–363. [DOI] [PubMed] [Google Scholar]
- 43.Jamal R A, Roughley P J, Ludwig M S. Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cell Mol Physiol 2001280306–315. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Miura S, Mori‐Abe A.et al Impact of menopause on the augmentation of arterial stiffness with aging. Gynecol Obstet Invest 200560162–166. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y X, Fitch R M. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol 20042379–384. [DOI] [PubMed] [Google Scholar]
- 46.Hjortdal J O. Biomechanical studies of the human cornea. Development and application of a method for experimental studies of the extensibility of the intact human cornea. Acta Ophthalmol Scand 199573364–365. [PubMed] [Google Scholar]
- 47.Koob T J, Jeffrey JJ Eisen A Z.et al Hormonal interaction in mammalian collagenase regulation. Comparative studies in human skin and rat uterus. Biochim Biophys Acta 19801713–23. [DOI] [PubMed] [Google Scholar]
- 48.Sato T, Ito A, Yamashita K, Hayakawa T. Hormonal regulation of collagenolysis in uterine cervical fibroblasts – modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases by progesterone. Biochem J 1991275645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]