Abstract
Impaired visual attention is a common manifestation of cerebral dysfunction. In adults, closed head trauma, cerebral microvascular ischaemia and dementia are common causes. In children, aetiologies include periventricular leukomalacia, hydrocephalus, hypoxic ischaemic encephalopathy and brain damage caused by hypoglycaemia. The resultant visual disability can be profound even when visual acuities are unaffected, and can cause significant disability in the execution of daily activities. This can prompt consultation with an eye care specialist. Patients complain of poor vision, difficulty in identifying someone in a group, or finding an object on a patterned background or among other objects, but a thorough examination often does not reveal the clinical basis for these complaints. The diagnosis of attentional dysfunction is also easily missed because at present it can only be recognised on the basis of adequate history taking from both the patient and close relatives and friends. The Useful Field of View test facilitates the detection and quantification of this disorder. Management includes the implementation of strategies that diminish background pattern and foreground clutter.
Keywords: impaired visual attention, useful field of view
Visual attention
There is a considerable body of literature on the subject of visual attention. A search of Medline currently reveals over 1600 hits, but few are to be found in the ophthalmic literature. While we are alert and awake, our minds are constantly receiving visual information. When looking at a scene, we are only aware of those elements we are paying attention to and those that distract our attention.1 The ability to survey a visual scene, locate and recognise an object of interest, and decide on an appropriate plan of action recruits a number of complex cognitive higher visual pathways. It is also constrained by the mechanisms of simultaneous perception and time.2 Visual sensory data pass from the eye to the primary visual or occipital cortex. Thereafter the information is processed in two principal locations, the temporal and the parietal lobes.3 The temporal lobes contain “image libraries” and bring about recognition of what is being looked at. The posterior parietal lobes appraise the entire visual scene and interact with the frontal lobes in choosing the object of interest and planning appropriate visually guided movement. Recent research suggests that from a functional point of view there are two pathways, the dorsal stream, which links the visual cortex with the parietal lobes, and the ventral stream, which links the visual cortex with the temporal lobes.4,5 The posterior parietal cortex contributes significantly to attentional visual function. Severe bilateral posterior parietal pathology gives rise to simultanagnosia in which there is profound difficulty registering the presence of any object that is not being attended to. Affected individuals have an inability to interpret the totality of the scene despite a preserved ability to apprehend individual portions of the whole. Natural visual scenes are cluttered and contain many different objects that cannot all be processed simultaneously. Therefore, from an operational perspective, visual attention is a matter of organising multiple brain centres to act in concert to select relevant and to filter out irrelevant information. Evidence from functional brain imaging suggests that attention operates at various processing levels within the visual system and beyond. The lateral geniculate nucleus (LGN) appears to be the first stage in processing visual information. In addition to retinal afferents, the LGN receives modulatory inputs from the striate cortex (mainly the V1 layer), the thalamic reticular nucleus (TRN) and the brainstem; it probably represents the first stage in the visual pathway at which cortical top‐down signals affect visual information processing.6 The TRN receives input from the LGN, V1, several extrastriate areas and the pulvinar. It probably also acts as a node where several cortical areas and thalamic nuclei interact to modulate the transmission of visual information further through the LGN.7 Second, intermediate cortical processing levels, such as the V4 and TEO (cytoarchitectonic area located in the inferotemporal and occipital cortex, ventral to area V4) areas of the visual cortex, are important sites where relevant visual information is selected and irrelevant information is filtered out.8,9 Third, the superior parietal lobule, frontal eye fields and supplementary eye fields serve as sources of top‐down feedback signals that modulate neural processing in the visual system.10,11,12 As a whole, this network mediates target selection and distractor suppression.13,14,15,16 Fourth, visual information from different cortical areas is integrated in the pulvinar of the thalamus. The visual maps in the pulvinar are arranged in such a way that neurons representing corresponding parts of the visual field in cortical visual areas project to similar parts of the pulvinar maps, thereby allowing the pulvinar to act as an integrator.17,18,19 The pulvinar can in turn be influenced by signals originating in the frontal and parietal eye fields, with the superior colliculus acting as an important link.20,21 The overall view that emerges is that neural mechanisms of selective attention operate at multiple stages in the visual system. In this respect, attention can be considered to be a multilevel selection process.6
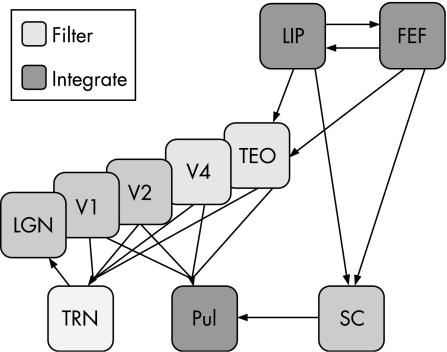
Figure 1 Neural architecture of visual attention. The schematic diagram illustrates the widely distributed networks of brain areas that subserve visual attention and operate across various processing levels. The lateral geniculate nucleus (LGN) is the first stage at which visual processing is modulated by attention; this modulation may be under control of the thalamic reticular nucleus (TRN), which operates as a local integrator of visual information (striped blue box). Intermediate cortical areas V4 and TEO act as filter sites to reduce the amount of unwanted information (green boxes). Higher order areas in the lateral intraparietal (LIP) area and frontal eye field (FEF) cortices integrate information from the visual system and provide top‐down attentional control via feedback connections (blue boxes). Furthermore, the pulvinar (Pul) may act as an additional integrator receiving information from both the visual system and the higher order areas via the superior colliculus (SC). The connectivity of these brain systems is indicated in simplified form and does not reflect the complexity of the known anatomical connections. It should be noted that most, if not all, of the connections are reciprocal. Reprinted from Kastner and Pinsk.6 Copyright 2004, with permission from Psychonomic Society, Inc.
These levels of attention can further be divided into subconscious and conscious visual processes. Loss of the striate cortex in both the human22 and monkey23 leaves elements of intact visual function, which in humans are at least ostensibly subconscious24 and primarily serve movement perception. Recent functional magnetic resonance imaging evidence indicates that brain activation caused by conscious attention can be detected in a variety of brain areas including V1.25,26 Such observations reinforce the work of animal studies, which indicate that persistent subcortical mechanisms mediated by the pulvinar and superior colliculus contribute to such subconscious visual function.27,28
Visual attention is not, however, an all or nothing phenomenon and there are many ways of both describing and quantifying it. Searching the visual scene involves both parallel and serial mechanisms.29,30,31 The capacity to move effortlessly through the visual world has recently been argued to be subconscious, reflexive and remarkably accurate. Such preattentive vision does not entirely entail conscious analysis of the visual world and is a global visual function providing simple analysis of the whole scene in a parallel fashion. Foveation on the other hand requires sequential serial attentive mechanisms for the conscious analysis of the visual world. Pre‐attentive vision is responsible for the phenomenon of “pop out” in which an element of the visual scene is sufficiently different from the background that it spontaneously stands out. Such differences include movement, colour and contrast. On the other hand, complex images such as faces and words require serial search to identify, and this constitutes a bottleneck in visual information processing, as this takes much longer to process.32,33
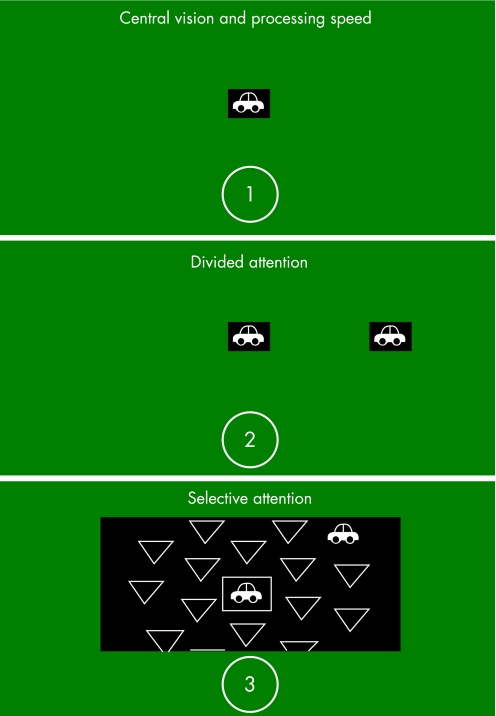
Figure 2 The first subtest is for central attention. It requires the identification of a silhouette of a car or truck presented in a central fixation box. The second subtest measures divided attention and involves identification of the central target along with localisation of a simultaneous peripheral target (silhouette of a car) presented at a fixed eccentricity of 12.5 cm from the central target, near the edge of the screen (at approximately 30° visual angle). The peripheral target is presented at one of eight locations along the cardinal and oblique axes. The subject is asked to identify the centrally presented object and to locate the direction of the peripheral target. The third subtest repeats these two tasks, but also includes additional visual distracters consisting of triangles or rectangles of the same size and luminance as the peripheral targets, which fill the rest of the visual display. Pictures of the Useful Field of View (UFOV) subtests are reprinted with permission of Visual Awareness, Inc. UFOV® is a registered trademark of Visual Awareness, Inc.
Profound cognitive visual impairment caused by parietal and temporal lobe pathology is rare, but minor dysfunction, especially as a result of aging is relatively common. There is a consensus among scientists and clinicians that the speed with which we process information gradually slows down with age. This may be as a result of the decreased speed of neural transmission.2 Older adults have greater difficulty than young people in carrying out everyday tasks that require visual search, peripheral visual attention and the extraction of information from cluttered visual scenes.34 Elderly people need visual information to be more conspicuous, presented for longer periods of time, or presented in isolation, for it to trigger an appropriate response. This slows down quick reactions, which are important for safety, such as in driving, crossing roads and accurately performing various daily activities. Children with impaired visual attention presenting to ophthalmologists have a characteristic symptom complex. This comprises difficulty seeing something pointed out in the distance, disability identifying a well‐known person in a group, problems finding an item of clothing in a pile and inability to find a chosen toy in a toy box without separating all the toys out.35 Visual neglect on one side, most commonly on the left, is occasionally seen in children as a sequel to unilateral, particularly right‐sided posterior parietal damage.
Clinical investigation of visual attention
Attention can be studied in many different ways. Most studies are conducted using customised stimuli and a methodology unsuitable for clinical practice. Certain tests have, however, been modified for use in a non‐research setting. Most of these are aimed at particular conditions, such as attention deficit/hyperactive disorder or dementia, and test attention across multiple modalities. A review of these is beyond the scope of this article. Many add a single test of visual attention to a battery of visual perception assessments. The most popular of these are discussed below.
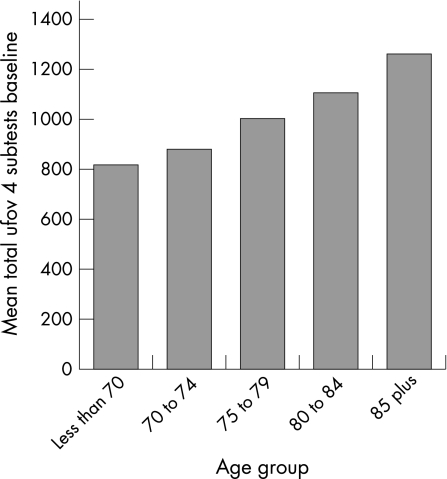
Figure 3 The graph represents the sum total score of all the Useful Field of View (UFOV) subtests by age group. Smaller scores reflect better performance. Reprinted from Edwards et al.43 Copyright 2006, with permission from Elsevier.
The Cookie Theft Picture36 taken from the Boston Diagnostic Aphasia Battery, is designed to have a balance of information in all four quadrants. Individuals with attentional disorders may not be able to describe the picture in a coordinated fashion. There may be an asymmetric perception of the scene in unilateral neglect. Qualitative assessment of the patient's verbal description of the diagram enables various attentional disorders such as hemifield neglect37 or Balint's syndrome38,39 to be identified. It has also been used in dementia40 and in perceptual defects after eclampsia.41 Exclusion criteria include aphasia sufficient to prevent verbal description and significant visual acuity or perimetric visual field loss.
The Line Bisection Task, first described by Best,42 involves the patient marking the middle of a number of horizontally drawn lines on a piece of paper. These vary in length and position. Information is gathered concerning how accurate the individual is and whether any lines are left unmarked. For example, those with left‐sided hemineglect may leave some lines unmarked on the left hand side and may also manifest a rightward bias when estimating and marking the mid‐point of a line. Similar findings have been reported in patients with frontal lobe lesions.44 Despite repeated findings of systematic bias among normal individuals,45 the test has been shown to be useful in detecting unilateral neglect.46,47
The Visual Exploration Test by Poppelreuter48 involves a large board hung vertically, on which numbers, letters and symbols are placed. The patient's task is to locate a particular target or group of targets. By observation of which ones are found, and in what timescale, the test provides information on attentional mechanisms. Many patients with neglect will be unable to find targets in their affected hemifield, despite being able to locate all four corners of the board. General attention can be tested by asking the participant to mark a subgroup of targets such as odd numbers. Some may be missed or the task may take a much longer time than usual. This test in particular has inspired many of the modern tests for attention currently available. Modern versions with validated normative data include the Gainotti Test and the Sky Search subtest in the Test of Everyday Attention in Children (Harcourt Assessment).
The Useful Field of View test (UFOV; Visual Resources, Inc., Chicago, Illinois, USA) developed by Ball and colleagues49,50,51,52 is usually performed binocularly, and measures the ability to process rapidly presented, increasingly complex information, within a restricted time period. Unlike conventional measures of visual field, which assess visual sensory sensitivity, this test also relies on higher‐order visual processing skills, such as selective and divided attention and rapid visual processing speed. It is assessed by means of computer‐based software and comprises three (or four in a few versions) increasingly difficult visual subtests, evaluating central, divided and selective attention. Usually a 17‐inch touch‐sensitive monitor is used to view the images, and participants are seated 40 cm from the screen. The targets measure approximately 2 cm2 each and subtend a visual angle of 3°. All targets are presented at progressively decreasing exposure durations, between 500 and 16 milliseconds, using a seven‐reversal staircase paradigm.
The fourth subtest36 is similar to the third subtest except that the central task is more demanding. Two targets are presented in the central fixation box (either two cars, two trucks, or one car and one truck) and the participants must indicate if the targets inside the box are the same or different. As in subtest 3, simultaneous localisation of a peripheral target is also required. Scores for each subtest are expressed as the display duration, in milliseconds, at which the participant performed accurately on 75% of the trials, using a seven‐reversal double staircase method. The scores of each test can range from 16 to 500 ms.
Poor performance in the UFOV test has being found to be a significant predictor of future at‐fault road traffic accidents.53,54
Management of impaired visual attention
Preliminary results suggest that the UFOV may be a useful tool for occupational therapists in retraining visual attention. When used as part of a battery of exercises, the UFOV has been shown to improve driving performance (for up to 18 months post‐training)55and the execution of instrumental activities of daily living56,57 in older adults. Stroke patients often have poor UFOV scores, indicating a substantial reduction in visual attention.58 UFOV performance has been found to improve significantly after retraining exercises with the software (by manipulating various parameters such as the colour of peripheral targets, luminance of distracters, duration of presentation of a target on the screen). It is uncertain, however, whether these positive changes represent improved overall visual attention or isolated learning of the UFOV tasks, and this is the subject of further investigation.
Recent research suggests that reading‐disabled individuals process visual information differently from normal readers as a result of a presumed magnocellular pathway deficit.59,60,61,62 In the light of this hypothesis, using the UFOV test, Edwards and Ball63 found that children with reading disability process visual information more slowly, are more easily distracted, and make more localisation errors, than children without such disabilities. Irrelevant peripheral information is more distracting in those subjects, resulting in poorer visual search skills and less effective allocation of visual attention.62 On the other hand, visual processing deficits play a relatively minor role in adult reading disability.64 Masking off adjacent text as well as using large print (to provide less crowding) can facilitate reading in adults with impaired visual attention. The role of the UFOV as a practice tool for improving reading skills has yet to be investigated.
In children, various coping strategies can improve visual attention. Diminishing background pattern and foreground clutter, along with the organisation of possessions assist in finding things. Friends/relatives need to understand that they will be identified more easily if standing alone and not in a group of people. To aid reading, text should be broken down into small parts and presented sequentially. Widening the gap between words, double spacing of printed text as well as covering words as they are read can be quite helpful. Some parents report that their child appears not to hear instructions if asked to do something when concentrating on another task, such as watching television. To communicate properly, the television had to be turned off or a favourite toy removed. Reduction in background noise also helps in improving attention.65
Conclusion
Visual attention is an important element of visual perceptual function. Attentional disorders occur commonly in young children, in the elderly and in patients with cerebral damage. A tendency to bump into obstacles and difficulty seeing, despite normal visual acuities, are common presenting complaints both for the young and old. Such symptoms can easily be dismissed when in fact they are far from trivial because they can be profoundly disabling and place both the patient and others at risk of injury. It is therefore important for ophthalmologists to be able to recognise the features of impaired visual attention.
Contemporary research shows that the UFOV test can be used to provide a rapid quantitative measure of visual attention and processing speed. It can also potentially be used as a part of speed of processing training. The remaining step is to apply this knowledge base in a reasonable and concise manner as a means of increasing the viability and safety of older adults in daily activities such as driving and navigation, and to help children improve performance at school and other educational and recreational activities.
Abbreviations
LGN - lateral geniculate nucleus
TRN - thalamic reticular nucleus
UFOV - Useful Field of View
Footnotes
Competing interests: None declared.
References
- 1.Boynton G M. Attention and visual perception. Curr Opin Neurobiol 200515465–469. [DOI] [PubMed] [Google Scholar]
- 2.Salthouse T A. The processing‐speed theory of adult age differences in cognition. Psychol Rev 1996103403–428. [DOI] [PubMed] [Google Scholar]
- 3.Dutton G N. Cognitive vision, its disorders and differential diagnosis in adults and children. Eye 200317289–304. [DOI] [PubMed] [Google Scholar]
- 4.Milner A D, Goodale M A. The visual brain in action. Oxford University Press: Oxford 1195
- 5.Stasheff S F, Barton J J. Deficits in cortical visual function. Ophthalmol Clin North Am 200114217–242. [PubMed] [Google Scholar]
- 6.Kastner S, Pinsk M A. Visual attention as a multilevel selection process. Cognit Affect Behav Neurosci 20044483–500. [DOI] [PubMed] [Google Scholar]
- 7.Guillery R W, Feig S L, Lozsadi D A. Paying attention to the thalamic reticular nucleus. Trends Neurosci 19982128–32. [DOI] [PubMed] [Google Scholar]
- 8.De Weerd P, Peralta MR I I I, Desimone R.et al Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat Neurosci 19992753–758. [DOI] [PubMed] [Google Scholar]
- 9.Kastner S, DeWeerd P, Desimone R.et al Mechanisms of direct attention in the human extrastriate cortex as revealed by functional MRI. Science 1998282108–111. [DOI] [PubMed] [Google Scholar]
- 10.Corbetta M, Shulman G L. Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 20023201–215. [DOI] [PubMed] [Google Scholar]
- 11.Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci 2000191–100. [DOI] [PubMed] [Google Scholar]
- 12.Kastner S, Ungerleider L G. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 200023315–341. [DOI] [PubMed] [Google Scholar]
- 13.Nobre A C. The attentive homunculus: now you see it, now you don't. Neurosci Biobehav Rev 200125477–496. [DOI] [PubMed] [Google Scholar]
- 14.Everling S, Tinsley C J, Gaffan D.et al Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci 20025671–676. [DOI] [PubMed] [Google Scholar]
- 15.Moore T, Armstrong K M. Selective gating of visual signals by micro stimulation of frontal cortex. Nature 2003421370–373. [DOI] [PubMed] [Google Scholar]
- 16.Schall J D, Thompson K G. Neural selection and control of visually guided eye movements. Annu Rev Neurosci 199922241–259. [DOI] [PubMed] [Google Scholar]
- 17.Adams M M, Hof P R, Gattass R.et al Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol 2000419377–393. [DOI] [PubMed] [Google Scholar]
- 18.Shipp S. Corticopulvinar connections of areas V5, V4, and V3 in the macaque monkey: a dual model of retinal and cortical topographies. J Comp Neurol 2001439469–490. [DOI] [PubMed] [Google Scholar]
- 19.Shipp S. The functional logic of cortico‐pulvinar connections. Philos Trans Roy Soc London. 2000;Series B 3581605–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benevento L A, Standage G P. The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J Comp Neurol 1983217307–336. [DOI] [PubMed] [Google Scholar]
- 21.Harting J K, Huerta M F, Frankfurter A J.et al Ascending pathways from the monkey superior colliculus: An autoradiographic analysis. J Comp Neurol 1980192853–882. [DOI] [PubMed] [Google Scholar]
- 22.Cowey A, Stoering P. The neurobiology of blindsight. Trends Neurosci 199114140–145. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey NK Vision in monkeys without striate cortex: a case study Perception. 1974;3:241–255. doi: 10.1068/p030241. [DOI] [PubMed] [Google Scholar]
- 24.Weiskrantz L. Blindsight: a case study and implications. Oxford: Oxford University Press 1998
- 25.Haynes J D, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci 20058686–691. [DOI] [PubMed] [Google Scholar]
- 26.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci 20058679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Hooser S D, Nelson S B. The squirrel as a rodent model of the human visual system. Vis Neurosci 200623765–778. [DOI] [PubMed] [Google Scholar]
- 28.Ouellette B G, Casanova C. Overlapping visual response latency distributions in visual cortices and LP‐pulvinar complex of the cat. Exp Brain Res 2006175332–341. [DOI] [PubMed] [Google Scholar]
- 29.Duncan J, Humphreys G W. Visual search and stimulus similarity. Psychol Rev. 1989;96;433–58. [DOI] [PubMed]
- 30.Wolfe J M. Visual search in continuous naturalistic stimuli. Vis Res 1994341187–1195. [DOI] [PubMed] [Google Scholar]
- 31.Connor C E, Egeth H E, Yantis S. Visual attention: bottom‐up versus top‐down. Curr Biol 200414R850–R852. [DOI] [PubMed] [Google Scholar]
- 32.Davis E T, Palmer J. Visual search and attention: an overview. Spatial Vis 200417249–255. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe J M, Horowitz T S. What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci 20045495–501. [DOI] [PubMed] [Google Scholar]
- 34.Kosnik W, Winslow L, Kline D.et al Visual changes in daily life throughout adulthood. J Gerontol 19884363–70. [DOI] [PubMed] [Google Scholar]
- 35.Dutton G N, Saaed A, Fahad B.et al The association of binocular lower visual field impairment, impaired simultaneous perception, disordered visually guided motion and inaccurate saccades in children with cerebral visual dysfunction – a retrospective observational study. Eye 20041827–34. [DOI] [PubMed] [Google Scholar]
- 36.Goodglass H, Kaplin E. The assessment of aphasia and related disorders, 2nd edn. Philidelphia: Lea & Febiger 1983
- 37.Kinsbourne M, Warrington E K. A disorder of simultaneous form perception. Brain 196285461–486. [DOI] [PubMed] [Google Scholar]
- 38.Coslett H B, Saffron E. Simultanagnosia: to see but not to see. Brain 19911141523–1545. [DOI] [PubMed] [Google Scholar]
- 39.Riddoch M J, Humphreys G W. Object identification in simultanagnosia: when wholes are not the sum of their parts. Cognit Neuropsychol 200421423–441. [DOI] [PubMed] [Google Scholar]
- 40.Mendez M F, Ashla‐Mendez M. Differences between multi‐infarct dementia and Alzheimer's disease on unstructured neuropsychological tasks. J Clin Exp Neuropsychol 199113923–932. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann M, Keiseb J, Moodley J.et al Appropriate neurological evaluation and multimodality magnetic resonance imaging in eclampsia. Acta Neurol Scand 2002106159–167. [DOI] [PubMed] [Google Scholar]
- 42.Best F. Uber Storungen der optischen lokalisation bei verletzungen und herderkranken im hunterhauptlappen. Neurol Cbl Lpz 191938427–432. [Google Scholar]
- 43.Edwards J D, Ross L A, Wadley V G.et al The useful field of view test: normative data for older adults. Arch Clin Neuropsychol 200621275–286. [DOI] [PubMed] [Google Scholar]
- 44.Silberpfennig J. Contributions to the problem of eye‐movements. III. Disturbances of ocular movements with pseudohemianopia in frontal lobe tumors. Confin Neurol 194141–13. [Google Scholar]
- 45.McCourt E. Performance consistency of normal observers in forced‐choice tachistoscopic visual line bisection. Neuropsychologia 2001391065–1076. [DOI] [PubMed] [Google Scholar]
- 46.Bisiach E, Bulgarelli C, Sterzi R.et al Line bisection and cognitive plasticity of unilateral neglect of space. Brain Cognit 1983232–38. [DOI] [PubMed] [Google Scholar]
- 47.Rorden C, Fruhmann Berger M, Karnath H O. Disturbed line bisection is associated with posterior brain lesions. Brain Res Ma. 2006, 29 108017–25. [DOI] [PubMed] [Google Scholar]
- 48.Poppelreuter W. Die pyschischen Schadigungen durch Kopfschuss im Kriege 1914/16. Mit besonderer Berucksichtigung der pathopsychologischen, padagogischen, gewerblichen und sozialen Beziehungen. Vol. 1, Die Storungen der neideren und hoheren Sehleistungen durch Verletzungen des Okzipitalhirns. Leipzig: Voss 1917
- 49.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age‐related declines in visual function. J Am Optometr Assoc . 1993;6471–79. [PubMed]
- 50.Ball K, Owsley C, Beard B. Clinical visual perimetry underestimates peripheral field problems in older adults. Clin Vis Sci 19905113–125. [Google Scholar]
- 51.Ball K, Owsley C, Sloane M E.et al Visual attention problems as a predictor of vehicle crashes in older drivers. Investig Ophthalmol Vis Sci 1993343110–3123. [PubMed] [Google Scholar]
- 52.Ball K K, Beard B L, Roenker D L.et al Age and visual search: expanding the useful field of view. J Optic Soc Am. A Optics, Image Sci Vision 198852210–2219. [DOI] [PubMed] [Google Scholar]
- 53.Ball K K, Roenker D L, Wadley V G.et al Can high‐risk older drivers be identified through performance‐based measures in a Department of Motor Vehicles setting? J Am Geriatr Soc 20065477–84. [DOI] [PubMed] [Google Scholar]
- 54.Myers R S, Ball K K, Kalina T D.et al Relation of useful field of view and other screening tests to on‐road driving performance. Percept Motor Skills 200091279–290. [DOI] [PubMed] [Google Scholar]
- 55.Roenker D L, Cissell G M, Ball K K.et al Speed‐of‐processing and driving simulator training result in improved driving performance. Hum Factors 200345218–233. [DOI] [PubMed] [Google Scholar]
- 56.Edwards J D, Wadley V G, Myers R S.et al Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology 200248329–340. [DOI] [PubMed] [Google Scholar]
- 57.Edwards J D, Wadley V G, Vance D E.et al The impact of speed of processing training on cognitive and everyday performance. Aging Mental Health 20059262–271. [DOI] [PubMed] [Google Scholar]
- 58.Mazer B L, Sofer S, Korner‐Bitensky N.et al Use of the UFOV to evaluate and retrain visual attention skills in clients with stroke: a pilot study. Am J Occupat Ther 200155552–557. [DOI] [PubMed] [Google Scholar]
- 59.Cornelissen P, Richardson A, Mason A.et al Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and control. Vis Res 1995351483–1494. [DOI] [PubMed] [Google Scholar]
- 60.Lovegrove W J, Bowling A, Babcock D. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science 1980210439–440. [DOI] [PubMed] [Google Scholar]
- 61.Talcott J B, Hansen P C, Willis‐Owen C.et al Visual magnocellular impairment in adult developmental dyslexics. Neuro‐ophthalmology 199820187–201. [Google Scholar]
- 62.Vidyasagar T R, Pammer K. Impaired visual search in dyslexia relates to the role of the magnocellular pathway in attention. Neuroreport 1999101283–1287. [DOI] [PubMed] [Google Scholar]
- 63.Edwards J D, Ball K K. The Useful Field of View in good versus poor readers. Investig Ophthalmol Vis Sci 199536S901 [Google Scholar]
- 64.Edwards J D, Walley A C, Ball K K. Phonological, visual and temporal processing in adults with and without reading disability. Reading and Writing:Interdiscipl J200316737–758. [Google Scholar]
- 65.McKillop E, Bennett D M, McDaid G.et al Problems experienced by children with cognitive visual dysfunction due to cerebral visual impairment – and the approaches which parents have adopted to deal with these problems. Br J Vis Impairm 200624121–127. [Google Scholar]