Abstract
Aim
To determine the incidence, methods of diagnosis, treatment strategies and outcomes for acute retinal necrosis (ARN) in the UK.
Methods
A 12‐month active case ascertainment study was carried out between March 2001 and March 2002 to record cases of ARN presenting to ophthalmologists via the British Ophthalmological Surveillance Unit (BOSU) reporting system. Questionnaires were sent to the reporting consultants, requesting data on patient characteristics, presentation, clinical findings, investigations and treatment. Diagnosis was made using the American Uveitis Society diagnostic criteria. Further questionnaires were sent at 2 weeks and 6 months to assess outcome and therapies.
Results
74 cases of ARN were reported by 58 consultants between March 2001 and March 2002. Questionnaires were returned for 49 cases (66.2%), of which 18 (36.7%) were excluded. Of the 31 cases included, 22 (71.0%) were male and 9 (29.0%) were female. The age range was 13 to 85 years (mean 54.3 years). 28 cases (90.3%) were unilateral, with 3 patients (9.7%) presenting with bilateral ARN.
An aqueous or vitreous biopsy was performed in only 18 patients, with one patient having both. Herpes viral DNA analysis was performed on all 19 biopsies, with identification of the viral DNA in 16; results from 3 biopsies were not documented. Varicella zoster virus (VZV) was the commonest cause identified in 10 patients (56%).
Of the 31 subjects, 27 (87.1%) were treated for ARN with systemic antiviral treatment: with intravenous antiviral in 23 cases (85.2%) and oral antiviral in 4 cases (14.8%). 21 of these patients went on to receive oral antiviral maintenance therapy. In addition to antiviral treatment, systemic steroids were given to 16 subjects (51.6%). Surgical intervention for retinal detachment was performed on 5 patients.
Conclusions
During the 12‐month study period, 31 cases of ARN met the diagnostic criteria set by the American Uveitis Society. The incidence in the UK based on this study is approximately 1 case per 1.6 to 2.0 million population per year. We have ascertained that the management of ARN throughout the UK is variable, suggesting that national guidelines would be of benefit.
Acute retinal necrosis (ARN) is characterised by confluent, peripheral, necrotising retinitis, peripheral occlusive arteritis and moderate‐to‐severe vitritis.1 ARN usually presents unilaterally and has a poor prognosis, however, bilateral cases have also been described.2,3 ARN is principally a clinical diagnosis, with a variable success rate of isolating the causative viral DNA with laboratory testing of aqueous or vitreous samples.1,2 Most reported cases have been caused by varicella zoster virus (VZV), accounting for 50% to 80% of cases, with herpes simplex virus (HSV) responsible for the remaining cases when an organism has been isolated.2,4,5,6
The incidence of ARN in the UK is unknown. It is possible that the incidence of ARN may be rising since both the number of immunosuppressed patients and the average age of the population in the UK are increasing.7 Prompt diagnosis and treatment is critical in attempting to reduce visual loss in this frequently blinding condition.
We have undertaken the first national population‐based study of ARN to assess the incidence, current methods of diagnosis, treatment strategies and outcomes in the UK.
Methods
A 12‐month active case ascertainment study was carried out between March 2001 and March 2002 to record cases presenting to UK ophthalmologists with ARN. This was conducted with the assistance of the British Ophthalmological Surveillance Unit (BOSU) via their yellow card reporting system. Questionnaires were sent to the reporting consultants, requesting data on patient characteristics, presentation, clinical findings at time of presentation, investigations and treatment. Further questionnaires were sent at 2 weeks and 6 months to assess outcome and therapies.
Cases were diagnosed using the American Uveitis Society Diagnostic Criteria (1994) (table 1).8
Table 1 American Uveitis Society diagnostic criteria (1994).
| • One or more foci of retinal necrosis in peripheral retina (± macula) with circumferential spread |
| • Evidence of occlusive vasculopathy |
| • Inflammatory reaction in vitreous and anterior chamber |
| • Not dependent on extent of retinal necrosis, gender, race, age, immune status |
Results
Patient characteristics
Seventy‐four cases of ARN were reported by 58 consultants, between March 2001 and March 2002. Questionnaires were returned for 49 cases (66.2%), of which 18 (36.7%) were excluded (because of double reporting, misdiagnosis and reporting error) (table 2). Thirty‐one cases (41.9%) were included in the study. No questionnaire responses were received for the remaining 25 cases originally reported to BOSU.
Table 2 Questionnaire response.
| Number of cases (%) | |
|---|---|
| Included | 31 (41.9) |
| Double reporting | 9 (12.2) |
| Mistaken diagnosis | 5 (6.8) |
| Reporting error | 4 (5.4) |
| No response | 25 (33.8) |
Of the 31 cases included, 22 (71.0%) were male and 9 (29.0%) were female. Caucasians accounted for 96.8% (30 cases). The age range was 13 to 85 years, with a mean age of 54.3 years. Twenty‐eight cases (90.3%) were unilateral, with the remaining three patients (9.7%) presenting with bilateral ARN (one patient with unilateral presentation went on to develop subsequent fellow eye involvement).
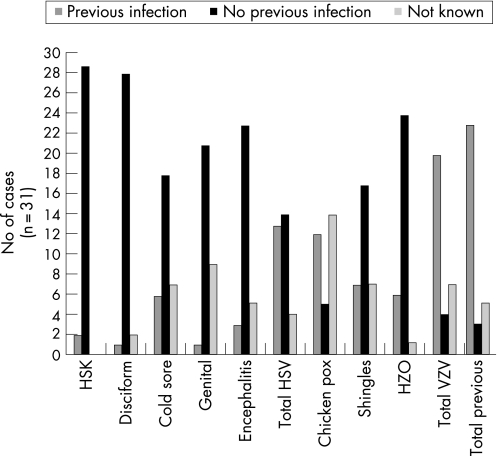
Previous herpetic ocular infections included herpes simplex keratitis and disciform keratitis (9.7%), and herpes zoster ophthalmicus (20.7%). Other HSV infections included cold sores (25.0%), genital ulcers (4.5%) and encephalitis (15.4%). Previous VZV infections included chickenpox (70.6%) and shingles (29.4%). Results are summarised in fig 1.
Figure 1 Herpes viral infections prior to acute retinal necrosis diagnosis.
Seven patients had pre‐existing acquired immunodeficiency: three secondary to iatrogenic immunosuppression post‐transplantation, three with iatrogenic immunosuppression for haematological malignancy, and one due to AIDS.
Presentation
The main presenting complaint was sudden visual loss (85.1%). Photophobia was reported in 54.5% of subjects, 25.8% complained of ocular pain and 26.1% of flu‐like symptoms. Only five patients presented with a red eye (16.1%).
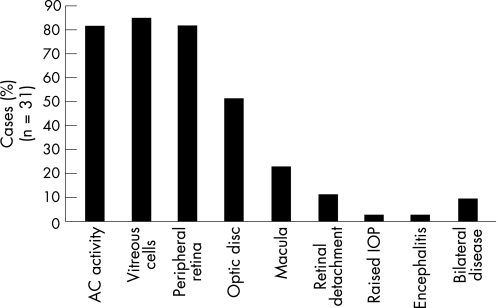
Of the 31 cases assessed, 25 (80.6%) had anterior chamber activity, 26 cases (83.9%) had vitreous cells, and 25 (80.6%) had peripheral retinal involvement (fig 2).
Figure 2 Signs at first presentation.
Investigations
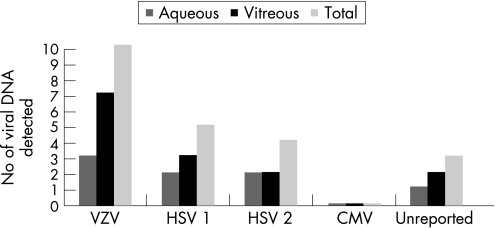
An aqueous and/or vitreous biopsy was performed in only 18 patients (58.1%). Only one patient had both an aqueous and vitreous tap. Thirteen vitreous biopsies (41.9%) and six aqueous taps (19.4%) were performed. Viral DNA analysis was performed on all 19 biopsies. The results from two patients (the first an aqueous and vitreous biopsy, and the second a vitreous biopsy), were not provided by the reporting consultants. Aqueous viral DNA analysis on the remaining five biopsies revealed one patient was positive for HSV‐1 and one for HSV‐2, two patients were positive for VZV, and the remaining subject was positive for HSV‐1, HSV‐2 and VZV. Vitreous PCR analysis on the remaining 11 biopsies revealed two patients positive for HSV‐1, one positive for HSV‐2, one positive for both HSV‐1 and HSV‐2, with the remaining seven patients positive for VZV. Cytomegalovirus was not detected by PCR analysis of aqueous or vitreous samples. The results are summarised in fig 3.
Figure 3 Ocular fluid analysis by PCR‐based assay for herpes virus.
Treatment
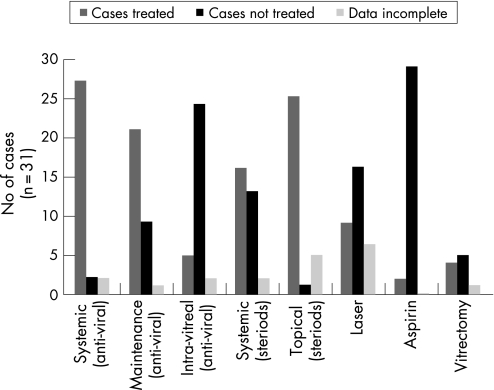
Treatment was found to be variable (summarised in fig 4). Of the 31 subjects included in the study, 27 patients (87.1%) were treated for ARN with systemic antiviral treatment. This systemic treatment was principally with intravenous antiviral in 23 cases (85.2%), and oral antiviral in the remaining four subjects (14.8%). Intravenous aciclovir was used in 21 cases, one subject received intravenous ganciclovir and the remaining patient was given cidofovir. Of the four patients treated with oral therapy, three received aciclovir and one received valaciclovir. Treatment for two cases was unreported. One patient did not receive any systemic treatment initially but was started on oral aciclovir on day 14 for a duration of 1 month, and the final subject initially received intravenous methylprednisolone for 3 days as the only systemic treatment and no systemic antivirals at the initial or later stages. Five patients received intravitreal foscarnet in addition to systemic intravenous aciclovir.
Figure 4 Acute retinal necrosis management.
Twenty‐one of these subjects then went on to receive oral antiviral maintenance therapy; 9 (42.8%) received aciclovir, 7 (33.3%) were prescribed valaciclovir and 5 (23.8.%) received famciclovir. The duration of maintenance therapy ranged from 5 days to 6 months. In addition to antiviral treatment, systemic steroids were given to 16 cases (51.6%). Steroids were given orally in 15 cases and intravenously in the remaining subject. At the initiation of treatment, 25 patients (80.6%) also received topical steroids. One case received a periocular steroid injection. Aspirin was only given to two subjects (6.5%) at the time of presentation.
Surgical intervention for retinal detachment was performed on five patients, ranging from day 14 to 6 months. Six patients underwent retinal barrier laser treatment at day 1, and three subjects were treated at day 14. Three of these subjects underwent further laser treatment at 6 months.
Both the dosages and duration of the various systemic antiviral treatments initiated varied between ophthalmology units. The total duration of antiviral therapy ranged from 5 days to 6 months. A wide range of treatment durations and dosages were also seen with steroid therapy; with treatment given initially for 2 weeks in 16 cases, and subsequently between 1 and 6 months in four of these 16 cases.
Complications
Complications occurred in 38.7% (12/31) of cases, with retinal detachment in nine out of the 12 cases (accounting for 75.0% of recorded complications). The time of retinal detachment varied from day 1 to 6 months. The treatment for retinal detachment also varied among institutions.
Other complications noted in the study included hypotony, cystoid macula oedema and rubeosis.
Outcome
At presentation 31 patients had visual acuity ranging from 6/5 to no perception of light (NPL). Unfortunately too few data were provided for visual acuity at day 14 or 6 months to accurately analyse the effectiveness of the various treatment regimens. The available data from day 14 and 6 months revealed that visual acuity in 44.1% (15/34) of eyes worsened and in 26.5% (9/34) of eyes it improved. Visual acuity subsequent to presentation was unreported for the remaining seven (22.5%) cases.
Only five out of the nine patients with retinal detachment had surgical intervention. In three of these five cases (60%) the vision following surgery deteriorated.
Discussion
During the 12‐month study period, 31 cases of ARN were ascertained that met the diagnostic criteria set by the American Uveitis Society. The incidence in the UK based on this study is a minimum of one case per 1.6 to 2.0 million population per year. This may represent an underestimation, due to reporting bias, data entry bias and case ascertainment bias. If all the cases reported to BOSU, including those with incomplete datasets, were used the incidence would have been 1.8 times greater.
It has been suggested that the incidence of ARN is likely to rise, due to an increasing number of immunocompromised subjects and an ageing population in many industrialised countries.12
ARN is a rare blinding disease characterised by rapidly progressive peripheral retinal necrosis. Early diagnosis and treatment is essential to improve the chances of preserving vision and preventing involvement of the fellow eye, since outcome is often poor.13,14 Although ARN is essentially a clinical diagnosis, in atypical cases or in clinical uncertainty PCR‐based assays should be undertaken urgently; however, treatment should not be delayed while awaiting laboratory confirmation. These assays have been widely used as an aid to the diagnosis and determination of the specific virus causing acute retinal necrosis.9,10,11,15,16 In this study viral DNA analysis was performed in 18 of the cases (58.1%); with a causative virus being identified in 16 (89%) of these patients. Our data confirm previous reports that VZV is the commonest cause of ARN. The at‐risk population for ARN are patients who have had previous zoster viral infections: chickenpox (70.6% of cases), shingles (29.2%) and zoster ophthalmicus (20.7%). The other risk factors identified in this study were previous herpes simplex cold sores (25%) and HSV encephalitis (15.4%), suggesting that in patients with presumed/confirmed HSV encephalitis it may be worth considering retinal screening.
We have ascertained that the management of ARN throughout the UK is extremely variable, suggesting that national guidelines would be of benefit. In a previous study initial therapy with intravenous aciclovir for a minimum of 2 weeks was used.17 This treatment would enable a high intraocular concentration to be achieved following which the patient can be maintained on an oral antiviral, ideally famciclovir, which has a better oral bioavailability.17,18
Involvement of the fellow eye can occur within the first 6 weeks, therefore Duker et al. and Blumenkranz et al. have recommended intravenous aciclovir initially followed by 4 to 6 weeks of oral aciclovir at a dosage of 2–4 g daily (there is no consensus on the optimal duration of antiviral therapy).19,20
A small pilot study has reported good outcomes with the sole use of oral antivirals, either famciclovir or valaciclovir.21 However, further larger studies are required to corroborate these findings.
Blumenkranz et al. advocate anti‐inflammatory therapy as an important component of ARN treatment since the reactive inflammatory response plays a significant role in tissue destruction.19 However, the concern remains of the possible adverse effect of oral steroids on enhancing viral replication, especially in the acute phase. It is therefore recommended to delay steroid use for 24–48 hours following the administration of aciclovir.19
There was a very low rate of aspirin use noted in the survey, despite the fact that antiplatelet therapy (heparin or aspirin) has been suggested as an adjunct in the treatment of the vaso‐occlusive component of ARN.22,23
Retinal barrier laser therapy to prevent rhegmatogenous retinal detachment (RRD) may be considered, although data on efficacy are lacking.23,24 Vitreo‐retinal surgery for retinal detachment may be useful,23,24 although patient outcome in our survey was poor, with 60% having a deterioration in vision following surgery. However, it remains possible that patients who develop RRD may have a more aggressive disease process with an associated worse prognosis.
Visual outcome in this devastating eye disease is often poor despite prompt diagnosis and treatment. The wide variability observed in our study regarding diagnostic methods and treatment paradigms suggests that national guidelines would be beneficial. The rarity of this disease hampers large‐scale studies, but a multi‐centre prospective randomised controlled trial would provide the best evidence‐based treatment options and management strategies.
Acknowledgements
We are grateful for the assistance of the Royal College of Ophthalmologists, the British Ophthalmological Surveillance Unit and to all reporting ophthalmologists in the UK (please refer to the addendum). Ms Jacqui Savage provided valuable administrative support.
Abbreviations
ARN - acute retinal necrosis
BOSU - British Ophthalmological Surveillance Unit
HSV - herpes simplex virus
RRD - rhegmatogenous retinal detachment
VZV - varicella zoster virus
Footnotes
Competing interests: None declared.
References
- 1.Urayama A, Yamada N, Saski T.et al Unilateral acute uveitis with retinal periarteritis and detachment. Japanese Journal of Clinical Ophthalmology 197125607–619. [Google Scholar]
- 2.Young N J A, Bird A C. Bilateral acute retinal necrosis. Br J Ophthalmol 197862581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willerson D, Aaberg T M, Reeser F H. Necrotising vasooclusive retinitis. Am J Ophthalmol 197784209–219. [DOI] [PubMed] [Google Scholar]
- 4.Ganatra J B, Chandler D, Santos C.et al Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol 2000129166–172. [DOI] [PubMed] [Google Scholar]
- 5.Van Gelder R N, Willig J L, Holland G N.et al Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology 2001108869–876. [DOI] [PubMed] [Google Scholar]
- 6.Itoh N, Matsumura N, Ogi A.et al High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol 2000129404–405. [DOI] [PubMed] [Google Scholar]
- 7.Holland G N. The progressive outer retinal necrosis syndrome. Int Ophthalmol 199418163–165. [DOI] [PubMed] [Google Scholar]
- 8.Holland G N, and the Executive Committee of the American Uveitis Society Standard diagnostic criteria for the acute retinal necrosis syndrome. Am J Ophthalmol 1994117663–667. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell S M, Fox J D, Tedder R S.et al Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis. J Med Virol 199443336–340. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham E T, Short G A, Irvine A R.et al Acquired immunodeficiency syndrome‐associated herpes simplex virus retinitis. Clinical description and use of a polymerase chain reaction‐based assay as a diagnostic tool. Arch Ophthalmol 1996114834–840. [DOI] [PubMed] [Google Scholar]
- 11.Knox C M, Chandler D, Short J A.et al Polymerase chain reaction‐based assays of the vitreous samples for the diagnosis of the viral retinitis. Ophthalmology 199810537–45. [DOI] [PubMed] [Google Scholar]
- 12.Pepose J S. The potential impact of the varicella vaccine and new antivirals on ocular disease related to varicella zoster vaccine. Am J Ophthalmol 1997123243–251. [DOI] [PubMed] [Google Scholar]
- 13.Tan J C H, Byles D, Stanford M R.et al Acute retinal necrosis in children caused by herpes simplex virus. Retina 200121344–347. [DOI] [PubMed] [Google Scholar]
- 14.Thompson W S, Culbertson W W, Smiddy W E.et al Acute retinal necrosis caused by reactivation of herpes simplex virus type 2. Am J Ophthalmol 1994118205–211. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein B E, Conrad D, Margolis T P.et al Cytomegalovirus‐associated acute retinal necrosis syndrome. Am J Ophthalmol 1997123257–258. [DOI] [PubMed] [Google Scholar]
- 16.De Boer J H, Verhagen C, Bruinenberg M.et al Serological and polymerase chain reaction analysis of intra‐ocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol 1996121650–658. [DOI] [PubMed] [Google Scholar]
- 17.Tornerup N R, Fomsgaard A, Nielsen N V. HSV‐1 induced acute retinal necrosis syndrome presenting with severe inflammatory orbitopathy, proptosis, & optic nerve involvement. Ophthalmology 2000107397–401. [DOI] [PubMed] [Google Scholar]
- 18.Luber A D, Flaherty J F., Jr Famciclovir for treatment of herpesvirus infections. Ann Pharmacother 199630978–985. [DOI] [PubMed] [Google Scholar]
- 19.Blumenkranz M S, Duker J S, D'Amico D J. Acute retinal necrosis. In: Albert DM, Jackobiec FA, eds. Principles and practice of ophthalmology: clincal practice. Volume 2. Philadelphia: WB Saunders, 1994, chapter 78.
- 20.Duker J S, Blumenkranz M S. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol 199135327–343. [DOI] [PubMed] [Google Scholar]
- 21.Aizman A, Johnson M W, Elner S G. Treatment of acute retinal necrosis syndrome with oral antiviral medication. Ophthalmology 2007114307–312. [DOI] [PubMed] [Google Scholar]
- 22.Ando F, Kato M, Goto S.et al Platelet function in bilateral acute retinal necrosis. Am J Ophthalmol 19839627–32. [DOI] [PubMed] [Google Scholar]
- 23.Culbertson W W, Atherton S S. Acute retinal necrosis and similar retinitis syndrome. Int Ophthalmol Clin 199333129–143. [DOI] [PubMed] [Google Scholar]
- 24.Palay D A, Sternberg P, Jr, David J.et al Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol 1991112250–255. [DOI] [PubMed] [Google Scholar]