Abstract
Aim
To determine whether the efficacy of re‐operation for idiopathic full‐thickness macular hole (FTMH) remaining open after initial surgery with internal limiting membrane (ILM) peeling is correlated with macular hole configuration as determined by optical coherence tomography (OCT), macular hole size, macular hole duration before the first operation, or type of tamponade (gas or silicone oil).
Methods
A retrospective consecutive interventional case series of 28 patients (28 eyes) with a persisting macular hole after vitrectomy, ILM peel, and gas tamponade. 28 patients underwent repeat surgery involving vitrectomy and gas (n = 15) or silicone oil tamponade (n = 12) or no tamponade (n = 1). Autologous platelet concentrate (n = 22), autologous whole blood (n = 1), or no adjuvant (n = 5) was used. Preoperative OCT was undertaken in all eyes. The main outcome measures were anatomical closure and improvement of best‐corrected visual acuity (BCVA).
Results
Anatomical closure was achieved in 19 of 28 eyes (68%). BCVA improved in 12 eyes, remained unchanged in nine, and worsened in seven. BCVA improved in 11 of 19 eyes with anatomical closure, and in one of eight eyes without closure. Anatomical closure and improvement of BCVA correlated with preoperative macular hole configuration on OCT, with higher rates of closure (18 of 20 eyes versus one of eight eyes, p = 0.001) and greater improvement of BCVA (p = 0.048) in eyes with a cuff of subretinal fluid at the break margin. Macular hole size, type of tamponade, macular hole duration before the first operation, or preoperative BCVA did not significantly correlate with visual or anatomical outcome.
Conclusion
Macular hole configuration seems to be a strong prognostic indicator of anatomical closure and may help identify those patients most likely to benefit from re‐operation.
Keywords: OCT, macular hole, retreatment
Surgical treatment of idiopathic full‐thickness macular hole (FTMH) by vitrectomy was first described by Kelly and Wendel1 in 1991. Since then, the surgical technique has been refined to improve the anatomical as well as the functional outcome. The current surgical approach consists of pars‐plana vitrectomy removing vitreoretinal traction from the fovea, and intraocular tamponade with gas. Many surgeons also remove the internal limiting membrane (ILM).2,3,4,5,6,7,8 Some authors have reported an increased rate of anatomical closure with ILM peeling but no difference in functional outcome,2,3 whereas others have reported an improvement of anatomical and functional outcome with ILM peeling.4,5 Although most authors now report anatomical closure rates greater than 80%2,3,4,5,6,7,8 a small number of holes remain open after initial vitrectomy.
The main goal of the present retrospective case series was to determine whether the efficacy of re‐operation for idiopathic FTMH remaining open after initial surgery with ILM peeling is correlated with macular hole configuration as determined by optical coherence tomography (OCT), macular hole size, macular hole duration before the first operation, or type of tamponade (gas or silicone oil).
A secondary goal was to determine the anatomical and functional results of repeat macular hole surgery.
Patients and methods
Study design and patients
The present exploratory investigation was a retrospective consecutive interventional case series. We reviewed the records of all patients with idiopathic FTMH who had undergone macular hole surgery with standard three‐port pars‐plana vitrectomy including indocyanine green‐assisted ILM peeling, adjunctive autologous platelet concentrate, autologous whole blood or no adjuvant, and gas tamponade, between 2002 and 2005 (n = 179). The anatomical closure rate after primary surgery was 85%. Twenty‐eight patients (20 women, eight men) with a mean age of 71 years who had undergone unsuccessful surgery were included in the study.
Surgical treatment
All included patients were re‐treated with standard three‐port pars‐plana vitrectomy by experienced surgeons. Ten of 28 patients underwent concomitant standard small‐incision phacoemulsification cataract surgery (table 1). To confirm complete previous removal of the ILM the continuous BSS irrigation was briefly stopped during the operation and less than 0.5 ml indocyanine green (Pulsion Medical AG, Munich, Germany) at a 0.1% concentration dissolved in glucose 5% was injected into the BSS‐filled globe just above the posterior pole. After less than one minute, the irrigation was re‐started and thus the dye was quickly washed out of the globe. A fluid–air exchange was performed and one drop of autologous platelet concentrate, autologous whole blood or no adjuvant was applied to the macular hole before routine closure with either 16% SF6 gas or silicone oil as tamponade agent.
Table 1 Patient data.
| Patient No. | Age | Sex | Macular hole duration before first operation | BCVA preop (logMAR) | BCVA postop (logMAR) | Follow‐up (months) | Lens preop | Lens postop | Macular hole size preop (μm) | Macular hole configuration preop | Macular hole status postop | Adjuvant | Tamponade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | F | 6 months | −0.70 | −1.00 | 8 | Pseudophakic | Pseudophakic | 713 | Without cuff | Open | Blood | Oil |
| 2 | 80 | F | −0.60 | −1.00 | 14 | Pseudophakic | Pseudophakic | 322 | Without cuff | Open | None | None | |
| 3 | 63 | F | −1.60 | −1.60 | 8 | Pseudophakic | Pseudophakic | 900 | Without cuff | Open | Platelet | Gas | |
| 4 | 67 | F | 4 months | −1.00 | −1.60 | 16 | Phakic | Pseudophakic | 374 | Without cuff | Open | None | Oil |
| 5 | 40 | F | 4 days | −1.10 | −0.80 | 11 | Phakic | Phakic | 818 | Without cuff | Open | Platelet | Gas |
| 6 | 72 | M | 5 months | −1.00 | −1.00 | 19 | Pseudophakic | Pseudophakic | 443 | Without cuff | Open | Platelet | Oil |
| 7 | 77 | F | −1.60 | −1.60 | 11 | Pseudophakic | Pseudophakic | 938 | Without cuff | Open | Platelet | Gas | |
| 8 | 75 | F | 12 months | −1.30 | −1.30 | 30 | Pseudophakic | Pseudophakic | 338 | Without cuff | Closed | Platelet | Gas |
| 9 | 74 | F | −1.30 | −1.00 | 11 | Pseudophakic | Pseudophakic | 613 | With cuff | Closed | Platelet | Gas | |
| 10 | 79 | M | −1.60 | −1.60 | 20 | Phakic | Pseudophakic | 644 | With cuff | Closed | Platelet | Gas | |
| 11 | 76 | M | 12 months | −1.10 | −0.60 | 6 | Phakic | Pseudophakic | 475 | With cuff | Closed | Platelet | Gas |
| 12 | 78 | F | 3 months | −1.00 | −0.40 | 14 | Pseudophakic | Pseudophakic | 815 | With cuff | Closed | Platelet | Gas |
| 13 | 71 | F | 2 weeks | −1.00 | −0.70 | 9 | Pseudophakic | Pseudophakic | 362 | With cuff | Closed | Platelet | Gas |
| 14 | 72 | F | 4 weeks | −0.70 | −0.70 | 16 | Phakic | Pseudophakic | 617 | With cuff | Closed | Platelet | Gas |
| 15 | 60 | F | 18 months | −0.80 | −0.40 | 16 | Pseudophakic | Pseudophakic | 339 | With cuff | Closed | Platelet | Gas |
| 16 | 81 | M | 6 months | −1.30 | −1.30 | 10 | Phakic | Pseudophakic | 650 | With cuff | Closed | None | Gas |
| 17 | 64 | F | 3 months | −1.60 | −1.60 | 6 | Phakic | Pseudophakic | 652 | With cuff | Closed | Platelet | Oil |
| 18 | 74 | F | 6 months | −1.00 | −0.90 | 28 | Phakic | Pseudophakic | 518 | With cuff | Closed | Platelet | Gas |
| 19 | 72 | M | 4 months | −0.70 | −0.60 | 10 | Phakic | Phakic | 300 | With cuff | Closed | Platelet | Gas |
| 20 | 67 | F | 12 months | −1.00 | −1.60 | 6 | Pseudophakic | Pseudophakic | 643 | With cuff | Closed | Platelet | Oil |
| 21 | 71 | F | 3 months | −1.00 | −1.30 | 34 | Phakic | Pseudophakic | 425 | With cuff | Closed | Platelet | Oil |
| 22 | 50 | M | 13 months | −0.90 | −0.60 | 36 | Phakic | Phakic | 506 | With cuff | Closed | Platelet | Oil |
| 23 | 62 | M | 2 weeks | −0.70 | −0.80 | 23 | Phakic | Pseudophakic | 270 | With cuff | Closed | None | Oil |
| 24 | 76 | F | −1.60 | −1.60 | 24 | Pseudophakic | Pseudophakic | 803 | With cuff | Open | None | Gas | |
| 25 | 72 | F | 3 months | −1.60 | −1.00 | 8 | Pseudophakic | Pseudophakic | 722 | With cuff | Closed | Platelet | Oil |
| 26 | 80 | M | 5 months | −0.70 | −0.50 | 12 | Pseudophakic | Pseudophakic | 383 | With cuff | Closed | Platelet | Oil |
| 27 | 77 | M | 2 months | −1.00 | −1.30 | 7 | Pseudophakic | Pseudophakic | 408 | With cuff | Open | Platelet | Oil |
| 28 | 68 | F | 2 months | −1.30 | −0.70 | 15 | Phakic | Pseudophakic | 686 | With cuff | Closed | Platelet | Oil |
BCVA, Best‐corrected visual acuity; logMAR, logarithm of the minimum angle of resolution. The macular hole of patient 14 closed after the second re‐operation, which was followed by another operation for retinal detachment. Patients 21 and 23 underwent additional surgery for retinal detachment after oil removal. Patients 2, 3, 7, 9, 10, and 24 did not give a clear history of macular hole duration before the first operation.
Study documentation
The following data were obtained: age; sex; pre and postoperative best‐corrected visual acuity (BCVA); lens status; macular hole duration before the first operation; length of time between the first operation and re‐operation; follow‐up after re‐operation; macular hole size and macular hole configuration as determined by OCT (OCT 1, Zeiss‐Humphrey; OCT 3, Zeiss‐Meditec, Jena, Germany); whether or not the ILM had been completely removed; type of adjuvant; and type of tamponade (gas or silicone oil).
Statistical analysis
The main outcome measures of this study were anatomical closure as assessed by OCT and improvement of BCVA. Using univariate descriptive statistics, our statistical analyses focused on identifying factors that would potentially influence anatomical closure and improvement of BCVA: macular hole configuration as determined by OCT, macular hole size, macular hole duration before the first operation, and type of tamponade (gas or silicone oil). We had no firm hypothesis as to which of these potential factors would contribute to explaining the variance in the main outcome measures. This was thus an exploratory rather than a confirmatory study and precautions for multiple testing were not taken. The Bonferroni method is not applicable in such cases.9
Descriptive statistics were reported as means, standard deviations, counts and percentages, when applicable. The following statistical tests were used: Fisher's exact test, Pearson's chi square test and Mann–Whitney test were used in order to group differences, the choice of the very test dependent on the scale character of the variable. All statistical analyses were conducted using SPSS 10.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
Mean follow‐up was 15.3 ± 8.6 months (mean ± SD; range 6–36 months; table 1). The interval between the first operation and re‐operation was 2.5 ± 0.96 months (mean ± SD; range 1–4 months).
Anatomical results
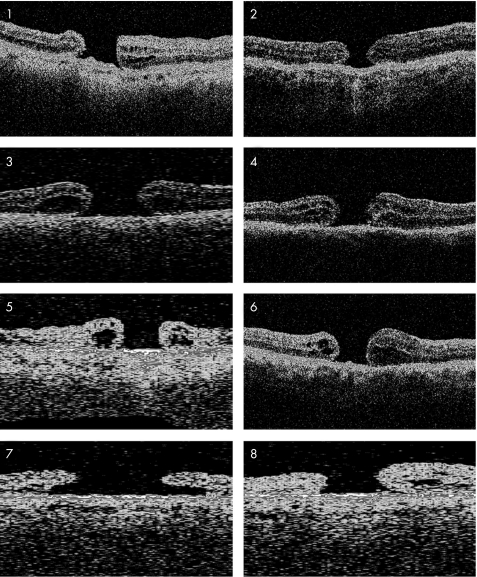
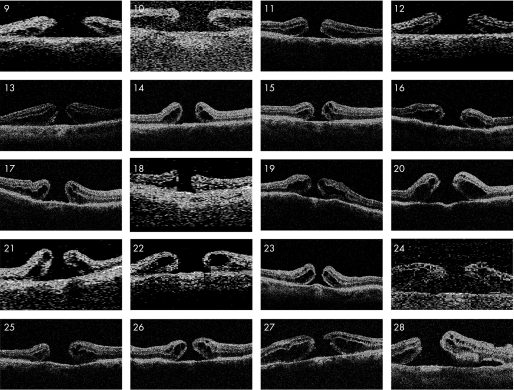
Anatomical closure was achieved in 19 of 28 eyes (68%; table 1). The ILM was noted to have been completely removed in the first operation in all eyes. Pre‐operative OCT revealed two distinct types of macular hole configuration. In the first type the hole appears flat and punched out without a distinct retinal cuff. We have termed the first type “without cuff” (fig 1). The second type was characterised by an elevated retinal cuff overlapping the hole. We have termed this type “with cuff” (fig 2). Anatomical closure was achieved in 18 of 20 eyes in the “with cuff” group but only in one of eight eyes in the “without cuff” group (p = 0.001, Fisher's test). Macular hole size, macular hole duration before the first operation, and type of tamponade (gas or silicone oil) were without statistically significant difference in the eyes with and without anatomical closure (p = 0.2, Mann–Whitney test; p = 0.4, Mann–Whitney test; and p = 0.3, Pearson's test, respectively).
Figure 1 Pre‐operative optical coherence tomography of scans of patients 1–8 showing a “without cuff” macular hole configuration characterised by the absence of a distinct retinal cuff of fluid. The hole appears flat and punched out. The macular hole configuration of patient 7 is atypical. Although there is a cuff this hole rather fits into the “without cuff” category because the cuff is small, it does not overlap the hole, and it is not elevated.
Figure 2 Pre‐operative optical coherence tomography scans of patient nos. 9–28. Macular hole configuration “with cuff” characterised by an obvious elevated retinal cuff overlapping the hole.
Functional results
The mean improvement in BCVA of all patients with the operation was 0.06 ± 0.33 (logarithm of the minimum angle of resolution; logMAR) (0.6 ± 3.3 Snellen lines). BCVA improved in 12 of 28 patients by at least one Snellen line, the mean improvement in BCVA of these patients was 0.33 ± 0.2 (logMAR) (3.3 ± 2 Snellen lines). BCVA remained unchanged in nine eyes. BCVA worsened in seven eyes by a mean of 0.37 ± 0.18 (logMAR) (3.7 ± 1.8 Snellen lines).
Preoperative BCVA was without statistically significant difference in the eyes with and without anatomical closure (p = 0.8, Mann–Whitney test). BCVA improved in 11 eyes, remained unchanged in five eyes, and worsened in three eyes with anatomical closure. BCVA improved in one eye, remained unchanged in four eyes, and worsened in four eyes without anatomical closure.
Improvement of BCVA was more likely in the “with cuff” group than in the “without cuff” group (p = 0.048, Fisher's test).
Complications
Retinal detachment that required surgical repair occurred in three patients. One detachment occurred after vitrectomy with gas and two after silicone oil removal (legend table 1).
Discussion
This study showed that re‐operation for failed macular hole surgery had a lower success rate than primary surgery, but that OCT could be used to help identify those patients most likely to have a favourable outcome. In particular, macular holes with a cuff of subretinal fluid at their margin were significantly more likely to close than those without this cuff of fluid.
Many factors may promote hole closure after surgery such as the relief of vitreous or ILM traction, and possibly the use of adjuvants. As vitreoretinal traction at the fovea and the ILM were both removed in the first operation, the persisting FTMH in the present study were presumably closed in response to intraocular tamponade, the adjuvant, or some other effect of surgery such as glial upregulation.10
It is not certain why those macular holes with a cuff of fluid were more likely to close than those without. Assuming that hole closure involves the centripetal movement of retinal tissue to occupy the foveal region, then the absence of an adhesion between the macular hole margin and the underlying retinal pigment epithelium might be expected to facilitate closure. Conversely, macular holes that appeared “stuck down” on OCT might be expected to have lower closure rates.
In most cases of failed primary macular hole surgery, the precise cause of failure is uncertain but it may be affected by multiple factors such as poor patient compliance with postoperative posturing regimens,1 long macular hole duration,1,3,4,11 and advanced macular hole stage.3,11 Intraoperative factors reported to enhance success include the use of adjuvants such as transforming growth factor‐beta 2,12 autologous serum13 or platelet concentrate.14 In addition, surgeons may experience a learning curve, with one group reporting anatomical closure rates of 88% in 2001,6 improving to 98% in 2004.7 Factors influencing primary hole closure may also influence the success rate of re‐operation of macular hole. Ezra et al.15 have described retreatment of persisting macular holes without previous ILM peeling. The authors performed epiretinal membrane dissection in 29 of 46 eyes after a first operation had failed. Anatomical closure was achieved in 80%. Ie et al.16 successfully retreated 12 of 12 macular holes with the application of growth factor and gas tamponade. Peeling of epiretinal membrane or of the ILM was not performed. The outcome of re‐operation for macular hole after an unsuccessful initial operation with ILM peeling has so far only been described in studies including small numbers of patients. Da Mata et al.7 achieved anatomical closure in two of three patients with silicone oil. Rizzo et al.17 successfully re‐operated on two patients using “heavy silicone oil” as a tamponade.
In the present study we did not find a statistically significant influence of macular hole size, pre‐operative BCVA, macular hole duration before the first operation, or type of tamponade (gas or silicone oil), but this may be explained by the relatively small number of patients. Large studies of repeat macular hole surgery are unlikely to be forthcoming because of the high success rates of primary surgery. In this case series, however, five patients had a long‐standing macular hole of 12 to 18 months duration before the first operation. All of these holes were closed with the second operation. Our observation that patients with long‐standing macular holes may be successfully operated (or re‐operated) is principally in accordance with others who successfully treated macular holes with a duration of symptoms of one to three years, with an anatomical closure rate of 70.8%.18 The weaknesses of this study include the retrospective nature of the data collection, non‐standardised surgical techniques and that the functional results may have been influenced by concomitant cataract surgery in 10 of 28 patients. Kusuhara et al.19 described a macular hole index defined as the ratio of the hole height to the base diameter, which refers to the perpendicular and horizontal dimensions of the hole visualized by OCT. They found that the visual outcome of primary macular hole surgery is positively correlated with a higher index that indicates a smaller horizontal and a greater perpendicular hole dimension. These findings of primary surgery may not be comparable to the results of re‐operation, however, we also found that a greater perpendicular hole dimension as seen in the holes “with cuff” seems to be a positive prognostic factor. Improved visualisation of the macular hole architectural morphology with ultrahigh resolution OCT20,21 and rapid serial fourier‐domain OCT22 have been described. New imaging techniques such as these may lead to a better understanding of macular hole formation and improve our ability to identify patients with a favourable surgical prognosis.
In conclusion, the results of this study show that OCT could help identify those patient with the greatest chance of surgical success of re‐operation for persisting macular hole. The study confirms that re‐operation for persisting macular hole has a reduced success rate compared with primary surgery. Repeat surgery in persistent “without cuff” macular holes may only be advisable in selected cases, for example in only‐eye situations.
Acknowledgements
The authors would like to thank the Centre for Clinical Trials at the University Hospital Regensburg for expert methodological and statistical advice.
Abbreviations
BCVA - Best‐corrected visual acuity
FTMH - full‐thickness macular hole
ILM - internal limiting membrane
OCT - optical coherence tomography
Footnotes
Presented in part at the Annual Meeting of the German Retina Society, Kiel, Germany, 9–10 June 2006
References
- 1.Kelly N E, Wendel R T. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 1991109654–659. [DOI] [PubMed] [Google Scholar]
- 2.Uemoto R, Yamamoto S, Aoki T.et al Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol 2002861240–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tognetto D, Grandin R, Sanguinetti G.et al Internal limiting membrane removal during macular hole surgery. Ophthalmology 20061131401–1410. [DOI] [PubMed] [Google Scholar]
- 4.Smiddy W E, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology 20011081471–1478. [DOI] [PubMed] [Google Scholar]
- 5.Kwok A K, Lai T Y, Yuen K S.et al Macular hole surgery with or without indocyanine green stained internal limiting membrane peeling. Clin Exp Ophthalmol 200331470–475. [DOI] [PubMed] [Google Scholar]
- 6.Da Mata A P, Burk S E, Riemann C D.et al Indocyanine‐ green‐assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology 20011081187–1192. [DOI] [PubMed] [Google Scholar]
- 7.Da Mata A P, Burk S E, Foster R E.et al Long‐term follow‐up of indocyanine green‐assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for idiopathic macular hole repair. Ophthalmology 20041112246–2253. [DOI] [PubMed] [Google Scholar]
- 8.Schaal K B, Bartz‐Schmidt K U, Dithmar S. [Current strategies for macular hole surgery in Germany, Austria and Switzerland] (In German). Ophthalmologe 2006103922–926. [DOI] [PubMed] [Google Scholar]
- 9.Perneger T V. What's wrong with Bonferroni adjustments. BMJ 19983161236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson T L, Hillenkamp J, Williamson T H.et al An experimental model of rhegmatogenous retinal detachment: surgical results and glial cell response. Invest Ophthalmol Vis Sci 2003444026–4034. [DOI] [PubMed] [Google Scholar]
- 11.Ryan E H, Gilbert H D. Results of surgical treatment of recent‐onset full‐thickness idiopathic macular holes. Arch Ophthalmol 19941121545–1553. [DOI] [PubMed] [Google Scholar]
- 12.Lansing M B, Glaser B M, Liss H.et al The effect of pars plana vitrectomy and transforming growth factor‐beta 2 without epiretinal membrane peeling in full‐thickness macular holes. Ophthalmology 1993100868–871. [DOI] [PubMed] [Google Scholar]
- 13.Liggett P E, Skolik S A, Horio B.et al Human autologous serum for the treatment of full‐thickness macular holes. A preliminary study. Ophthalmology 19951021071–1076. [DOI] [PubMed] [Google Scholar]
- 14.Paques M, Chastang C, Mathis A.et al Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double‐masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology 1999106932–938. [DOI] [PubMed] [Google Scholar]
- 15.Ezra E, Aylward W G, Gregor Z J. Membranectomy and autologous serum for the retreatment of full‐thickness macular holes. Arch Ophthalmol 19971151276–1280. [DOI] [PubMed] [Google Scholar]
- 16.Ie D, Glaser B M, Thompson J T.et al Retreatment of full‐thickness macular holes persisting after prior vitrectomy. A pilot study. Ophthalmology 19931001787–1793. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo S, Belting C, Genovesi‐Ebert F.et al Successful treatment of persistent macular holes using “heavy silicone oil” as intraocular tamponade. Retina 200626905–908. [DOI] [PubMed] [Google Scholar]
- 18.Scott R A, Ezra E, West J F.et al Visual and anatomical results of surgery for long standing macular holes. Br J Ophthalmol 200084150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusuhara S, Teraoka M F, Fuji S.et al Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol 2004138709–716. [DOI] [PubMed] [Google Scholar]
- 20.Ko T H, Fujimoto J G, Duker J S.et al Comparison of ultrahigh‐ and standard‐resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology 20041112033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko T H, Witkin A J, Fujimoto J G.et al Ultrahigh‐resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol 2006124827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam S, Zawadzki R J, Choi S.et al Clinical application of rapid serial fourier‐domain optical coherence tomography for macular imaging. Ophthalmology 20061131425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]