Abstract
Introduction
The inadvertent intra‐ocular administration of benzalkonium chloride‐preserved hydroxypropyl methylcellulose during cataract surgery at another hospital in 1999 resulted in toxic corneal endothelial injury and profound postoperative corneal oedema as a result of endothelial decompensation. The long‐term effect of this adverse event was assessed.
Methods
All 19 patients were invited to return for examination including corneal endothelial specular microscopy and pachymetry seven years after the incident. Results were compared with data from one year after the incident.
Results
Five patients attended for examination, one had received a penetrating keratoplasty and was, therefore, excluded. Ten patients had died and four had moved out of the region and were unable to attend. All four study patients were pain free and achieved 6/12 or better. Mean central corneal thickness reduced by 13% from 652.6 μm at one year to 563.4 μm. Mean central corneal endothelial cell density (n = 3) increased 28% from 663.7 cells/mm2 at one year to 835.7 cells/mm2 (p<0.05).
Conclusions
After toxic injury, corneal endothelial function may have a remarkable capacity for recovery even after the first postoperative year. The rise in central endothelial cell density may represent cell migration from less affected areas or cellular proliferation. Should this unfortunate event recur, clinicians may expect continued recovery beyond one year.
Keywords: corneal endothelium, injury, pachymetry, specular microscopy, cataract surgery, benzalkonium chloride
In February 1999 19 patients underwent phacoemulsification cataract surgery at another hospital, with the inadvertent intraocular use of hydroxypropyl methylcellulose (HPMC) 2% preserved with benzalkonium chloride 0.01%. The surgery was complicated by severe postoperative corneal oedema in all patients, with two requiring penetrating corneal transplants. Fourteen to 16 months after the cataract surgery 16 of the patients underwent examination including corneal endothelial specular microscopy and central corneal thickness measurement.1 At that assessment all patients were symptomatic, with either glare, blurred vision (table 1) or discomfort.
Table 1 Demographic data and visual results in 2000.
| Case no. (2000) | Sex | Age | Eye | Follow‐up (months) | Pre‐op VA | 1st Post‐op VA | VA at study in 2000 | Case no (2005) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 64 | R | 14 | CF | HM | 6/12 | |
| 2 | F | 74 | R | 14 | 3/60 | CF | 6/18 | |
| 3 | M | 78 | L | 14 | 6/12 | HM | 6/9 | |
| 4 | M | 82 | R | 14 | 6/18 | HM | 6/18 | |
| 5 | F | 88 | L | 15 | 6/9 | CF | 6/6 | |
| 6 | M | 78 | R | 15 | 6/9 | HM | 6/9 | |
| 7* | F | 67 | L | 15 | 6/6 | CF | 6/6 | 1 |
| 8* | F | 80 | R | 15 | 6/60 | HM | 6/9 | 2 |
| 9 | F | 79 | L | 15 | 6/9 | CF | 6/9 | |
| 10 | F | 90 | L | 15 | CF | CF | 6/18 | |
| 11 | M | 82 | R | 15 | 6/9 | CF | 6/6 | |
| 12 | F | 98 | L | 15 | 6/18 | 6/36 | 6/12 | |
| 13* | F | 79 | L | 16 | 6/24 | HM | 6/12 | 3 |
| 14 | F | 85 | R | 16 | 6/18 | CF | 6/12 (PK) | |
| 15 | M | 74 | R | 16 | 6/12 | HM | 6/9 (PK) | |
| 16* | F | 81 | R | 16 | 6/9 | CF | 6/60 | 4 |
CF, Counting fingers; HM, hand motion; PK, penetrating keratoplasty; VA, visual acuity. Age is given as at the time of surgery. *Patients included in the present 7‐year follow‐up study.
Mean central corneal thickness was 620 μm ± 71.1 (range 537–775, median 610) compared with a fellow eye mean of 563 μm ± 48.1 (range 516–670, median 562). Mean endothelial cell density was 830 cells/mm2 ± 280.8 (range 503–1392, median 737) compared with a fellow eye mean of 2017 cells/mm2 ± 446.5 (range 1244–2820, median 1950).
In order to study the long‐term effect of intra‐ocular benzalkonium chloride on the corneal endothelium we recalled all 19 patients in December 2005 (six years and 10 months after the incident) for a complete ophthalmic examination including central corneal thickness measurement and corneal endothelial specular microscopy.
Methods
Of the 19 patients, 10 had died, four had moved out of the region and were unable to attend and one attendee had received a penetrating keratoplasty and was, therefore, excluded from the study. The remaining four patients underwent a thorough examination including corneal endothelial specular microscopy and pachymetry. The four patients are numbered 1 to 4 in this study but correspond to patients 7, 8, 13 and 16 in the original article published in 2000 (table 1).
Pachymetry
Central corneal thickness was measured with an ultrasound pachymeter (Pachette; DGH Technology, Exton, PA, USA) after the installation of proxymethacaine 0.5% drops. Three measurements were taken and an average value was calculated. Comparison was made with central corneal measurements from 2000 when a different ultrasound pachymeter (Model Pocket; Quantel Medical Biovision International, France) was used. The fellow eye was used as a control.
Endothelial studies
Specular microscopy of the corneal endothelium was carried out using a non‐contact, semi‐automated specular microscope (Topcon, model SP‐2000P, Tokyo, Japan). Several photomicrographs of the central cornea were taken and the one with the best clarity was used for analysis. The maximum number of well‐defined endothelial cells were counted within each frame (range 16–41 cells) and cell parameters were determined having checked all cell contours. The central endothelial cell density, mean cell area and coefficient of variation in cell size were calculated and compared with data from 2000 (same model of specular microscope). The fellow eye was used as a control.
Statistical analysis
Data are presented with standard deviations (SD). The sample size was small and the data did not appear normally distributed. Therefore paired (Wilcoxon signed rank) and unpaired (Mann–Whitney U) non‐parametric tests were used to compare values from 2000 with 2005 and study and fellow eyes, respectively. Pachymetry data presented in Appendix 1 were analysed with unpaired t‐tests. Significance was assigned to the p<0.05 level.
Results
Demographic characteristics
All four patients were women. The mean age at the beginning of December 2005 was 80.8 years. Three of the four patients had had uneventful phacoemulsification cataract surgery in the other eye (all before benzalkonium chloride exposure). Two patients had mild, dry age‐related macular degeneration and one had open‐angle glaucoma, all of which pre‐dated the cataract surgery.
Symptomatology and visual acuity
All four patients had pain and/or photophobia in the affected eye in 2000. In 2005 all patients were pain free, although one patient was still experiencing glare on occasions. Best corrected Snellen visual acuity improved by four lines in two patients and two lines in one patient, but had dropped by one line in one patient. All patients achieved 6/12 or better (table 2).
Table 2 Best corrected visual acuities in 2000 (one year after surgery) and December 2005.
| 2000 | 2005 | |
|---|---|---|
| Patient 1 | 6/6 | 6/9 |
| Patient 2 | 6/9 | 6/5 |
| Patient 3 | 6/24 | 6/6 |
| Patient 4 | 6/60 | 6/12 |
Central corneal thickness
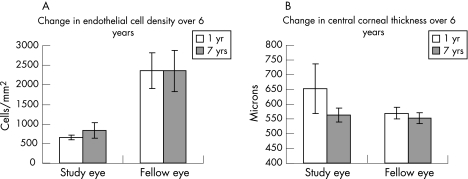
Patient 4 had residual Descemet's membrane folds but none of the other patients had clinical signs of corneal thickening. Corneal thickness was reduced compared with previous data in all patients (table 3, figure 1). Mean central corneal thickness reduced by 12.9% from 652.6 μm (83.9) in 2000 to 563.4 μm (22.8) in 2005. Fellow eye mean central corneal thickness was found to have reduced by 3.1% from 569.7 μm (20.7) to 551.8 μm (18.1), representing a consistent minor discrepancy between the pachymeters used in 2000 and 2006. The mean change in central corneal thickness was significantly greater in study eyes than fellow eyes (−89.1 μm versus −17.9 μm, p<0.05).
Table 3 Central corneal thickness (μm) in 2000 and 2005.
| Study eye | Fellow eye | |||||||
|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | Change | % | 2000 | 2005 | Change | % | |
| Patient 1 | 589.0 | 543.0 | −46.0 | −7.8 | 567.6 | 546.7 | −20.9 | −3.7 |
| Patient 2 | 610.6 | 576.3 | −34.3 | −5.6 | 599.0 | 578.7 | −20.3 | −3.4 |
| Patient 3 | 635.4 | 545.3 | −90.1 | −14.2 | 550.6 | 540.7 | −9.9 | −1.8 |
| Patient 4 | 775.2 | 589.0 | −186.2 | −24.0 | 561.6 | 541.0 | −20.6 | −3.7 |
| Mean | 652.6 | 563.4 | −89.1 | −12.9 | 569.7 | 551.8 | −17.9 | −3.1 |
| SD | 83.9 | 22.8 | 69.0 | 8.3 | 20.8 | 18.2 | 5.4 | 0.9 |
Figure 1 Comparison of endothelial cell density (A) and central corneal thickness (B) at one year and seven years after the incident in study and fellow eyes.
Specular microscopy
Patient 4 was excluded from analysis (no data in 2000 as a result of corneal oedema precluding imaging of the endothelium). Mean endothelial cell density for the three remaining patients rose by 28.1% from 663.7 cells/mm2 (59.9) in 2000 to 835.7 cells/mm2 (202.0) in 2005 (did not reach significance), whereas in the fellow eye endothelial counts remained stable with a mean of 2356.0 cells/mm2 (531.5) (table 4, figure 1).
Table 4 Endothelial cell density (cells/mm2) in 2000 and 2005.
| Study eye | Lens | Fellow eye | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECD 2000 | ECD 2005 | Δ | % Δ | CV 2000 | CV 2005 | ECD 2000 | ECD 2005 | Δ | % Δ | CV 2000 | CV 2005 | ||
| Patient 1 | 713 | 607 | −106 | −14.9 | 56 | 32 | ps | 2348 | 2291 | −57 | −2.4 | 31 | 33 |
| Patient 2 | 681 | 910 | 229 | 33.6 | 23 | 39 | ph | 2820 | 2917 | 97 | 3.4 | 32 | 28 |
| Patient 3 | 597 | 990 | 393 | 65.8 | 28 | 31 | ps | 1920 | 1860 | −60 | −3.1 | 47 | 35 |
| Patient 4 | n/a | 830 | n/a | n/a | n/a | 30 | ps | 1889 | 2037 | 148 | 7.8 | 27 | 28 |
| Mean | 663.7 | 835.7 | 172.0 | 28.2 | 35.7 | 33.0 | 2362.7 | 2356.0 | −6.7 | −0.7 | 34.2 | 31.0 | |
| SD | 59.9 | 202.0 | 254.3 | 40.6 | 17.8 | 4.1 | 450.2 | 531.5 | 89.8 | 3.6 | 8.8 | 3.6 | |
CV, Coefficient of variation in cell size; Δ, change in endothelial cell density (ECD); % Δ, percentage change in ECD; ph, phakic; ps, pseudophakic. Patient 4 (no data in 2000) was not included in mean and standard deviation calculations.
Discussion
The results demonstrate that in the event of profound damage and functional decompensation after toxic injury, the corneal endothelium has an impressive capacity for continued recovery beyond one year, with a return to normal central corneal thickness and consequent resolution of pain and improvement in visual acuity. Of the three patients with complete specular microscopy data, two had a prodigious rise in central endothelial cell density and all four patients had continued thinning of the central cornea between 14 months and seven years postoperatively. Evidence of central corneal thickening was present in only one patient, and visual acuity was 6/12 or greater in all the patients. Should such an inopportune event occur again in the future, clinicians may be able to offer some hope to affected patients that spontaneous long‐term recovery in endothelial function may occur.
It has been recognised that variation in corneal thickness may affect specular microscopy measurements through a magnification effect that could lead to an overestimation of cell density in corneas thinned by excimer laser.2 Theoretical calculations estimate this effect would be of the order of a 1–2% error in non‐contact specular microscopy for a 200 μm change in thickness. One of our patients exhibited a 65.8% increase in central endothelial cell density, whereas the fellow eye fell by 3.1%, and although our patient numbers are low, it seems unlikely that magnification error accounts for the observed rise in endothelial cell count. Furthermore, such delayed corneal endothelial recovery has previously been documented after exposure of the endothelium to a toxic detergent residue during cataract surgery.3
This leaves us to question the cause of the observed central endothelial population recovery after toxic insult when the natural history is for a steady decline in cell numbers at a rate of approximately 0.6% per year.4 There appears to be no such endothelial recovery after uneventful cataract surgery, which is itself subject to approximately 10% endothelial loss at one year after both phacoemulsification and extracapsular surgery.5 Numa et al.6 showed a progressive decline in endothelial counts after extracapsular cataract extraction with posterior chamber implants with an overall rate of loss 10 times greater than a control group over a five year period.
Recovery of central endothelial cell density must, intuitively, be as a result of cellular migration from the peripheral cornea or endothelial cellular proliferation. Although both these phenomena have been demonstrated after benzalkonium injury to the rabbit cornea in vivo,7 it has long been thought that the human response to corneal endothelial injury involved little or no cellular mitosis but relied solely upon migration and increased cellular size to compensate for cell loss.8Ex vivo human corneal endothelial cells may be readily induced to proliferate in vitro,9 and more recently studies have suggested that human corneal endothelium retains its proliferative capacity in vivo although this is inhibited under normal conditions.10
For migration without proliferation to account for the replacement of central endothelial cell loss implies a relative sparing of more peripheral areas from the toxic injury. Whereas the central endothelium may be inherently more susceptible to injury than the periphery, it is also quite conceivable that the endothelium at the central vertex of the cornea has more prolonged exposure to viscoelastics such as HPMC during cataract surgery because more peripheral areas, particularly near the main incision, are closer to the phacoemulsification and aspiration instruments. This would, therefore, lead to preferential central corneal endothelial toxic injury from benzalkonium‐preserved HPMC, thus allowing the relatively spared peripheral endothelial cells to migrate towards the centre to compensate for localised loss. Although peripheral endothelial specular microscopy was not performed at either time, a retrospective analysis of regional pachymetry results 14–16 months postoperatively revealed that although central corneal thickness was significantly greater in affected eyes (620 μm) than fellow eyes (563 μm, p<0.005), the peripheral corneal thickness was essentially the same (see Appendix 1). This supports the notion that, in this circumstance, the central corneas were preferentially affected, with possible sparing of the peripheral endothelium.
Whether migration or proliferation (or both) are responsible for the apparent repopulation of the central endothelium, this paper highlights the recuperative ability of the cornea after injury and may support the possible existence of a population of adult human corneal endothelial stem cells.
Abbreviations
HPMC - Hydroxypropyl methylcellulose
APPENDIX 1
Mean central and mean peripheral (superior, temporal, inferior and nasal) pachymetry readings in 2000 are detailed in table A1, showing preferential swelling of the central cornea and peripheral sparing when compared with the fellow eyes. Peripheral pachymetry readings (Model Pocket, Quantel Medical Biovision International) were taken using 12, 3, 6 and 9 o'clock positions, and these values were averaged to give a mean peripheral corneal thickness for each of the 13 patients undergoing regional pachymetry in 2000. No differences between peripheral regions were found with respect to corneal thickness, and in particular the superior peripheral cornea (closest to the main section) was not significantly different to other peripheral regions.
Table A1 Mean central and mean peripheral pachymetry readings in 2000.
| Central pachymetry (n = 16) | Peripheral pachymetry (n = 13) | |
|---|---|---|
| Study eye | 620 | 1060 |
| Fellow eye | 563 | 1066 |
| Unpaired t‐test | p<0.005 | p = 0.74 |
Footnotes
Competing interests: None.
References
- 1.Eleftheriadis H, Cheong M, Sandeman S.et al Corneal toxicity secondary to inadvertent use of benzalkonium chloride preserved viscoelastic material in cataract surgery. Br J Ophthalmol 200286299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isager P, Hjortdal J O, Ehlers N. Magnification changes in specular microscopy after corneal refractive surgery. Acta Ophthalmol Scand 199977391–393. [DOI] [PubMed] [Google Scholar]
- 3.Nuyts R M, Boot N, van Best J A.et al Long term changes in human corneal endothelium following toxic endothelial cell destruction: a specular microscopic and fluorophotometric study. Br J Ophthalmol 19968015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne W M, Nelson L R, Hodge D O. Central corneal endothelial cell changes over a ten‐year period. Invest Ophthalmol Vis Sci 199738779–782. [PubMed] [Google Scholar]
- 5.Bourne R R, Minassian D C, Dart J K.et al Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology 2004111679–685. [DOI] [PubMed] [Google Scholar]
- 6.Numa A, Nakamura J, Takashima M.et al Long‐term corneal endothelial changes after intraocular lens implantation. Anterior vs posterior chamber lenses. Jpn J Ophthalmol 19933778–87. [PubMed] [Google Scholar]
- 7.Nakahori Y, Katakami C, Yamamoto M. Corneal endothelial cell proliferation and migration after penetrating keratoplasty in rabbits. Jpn J Ophthalmol 199640271–278. [PubMed] [Google Scholar]
- 8.Mishima S. Clinical investigations on the corneal endothelium. Ophthalmology 198289525–530. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Joyce N C. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci 2004451743–1751. [DOI] [PubMed] [Google Scholar]
- 10.Joyce N C. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 200322359–389. [DOI] [PubMed] [Google Scholar]