Abstract
Aim
Using the anterior segment optical coherence tomography (AS‐OCT) to quantify changes in anterior segment morphology going from light to dark and following laser iridotomy (LI).
Methods
Prospective observational study. 17 consecutive subjects without peripheral anterior synechiae undergoing LI were evaluated using gonioscopy and AS‐OCT. Angle configuration including angle opening distance (AOD) at 500 microns anterior to the scleral spur, AOD500, trabecular‐iris space area up to 750 microns from the scleral spur, TISA750 and the increase in angle opening going from dark to light conditions was determined.
Results
Both mean AOD500 and TISA750 increased nearly threefold going from dark to light. Both also significantly increased following LI (p<0.001) as did gonioscopic grading of the angle in all quadrants (p<0.001, McNemar's test). Angles were more than twice as wide on average in the dark after LI than before LI (p<0.05). Both the mean absolute change and the mean proportionate change in AOD500 and TISA750 when going from light to dark were greater after LI than before (p<0.05).
Conclusion
Increased illumination as well as LI resulted in significant widening of the anterior chamber angle. AS‐OCT (which does not require a water bath and can be performed with the patient at the slit lamp) identified similar magnitude changes as those previously reported using ultrasound biomicroscopy (UBM). Furthermore, the angle appears to open more both in absolute terms and and proportionate terms in response to illumination after LI.
Primary angle‐closure glaucoma (PACG) causes substantial vision loss in Asia1,2,3,4,5,6 and is a leading cause of blindness worldwide.7 Laser iridotomy (LI) is the standard first‐line intervention in both acute and chronic forms of angle closure where the primary underlying mechanism is pupil block.8 It prevents the recurrence of acute attacks and virtually eliminates the risk of an acute attack in the fellow eye.9,10 In chronic PACG it can reverse appositional angle closure sufficiently to control the intraocular pressure (IOP).11,12 LI eliminates pupil block, allowing the convex iris to flatten and widening the anterior chamber angle. Increased illumination has similar effects on angle opening due to miosis‐induced iris flattening, however, the central iris tends to flatten after LI, but does not do so when going from dark to light conditions in the absence of a LI.13
Previous studies have documented these changes in anterior chamber angle morphology using ultrasound biomicroscopy (UBM) and gonioscopy. Gonioscopy is difficult to perform in a reproducible fashion, limiting the ability to quantify changes post‐LI. UBM gives reproducible images of the cross‐sectional anterior chamber angle anatomy, with very high resolution14 but requires a water bath on the eye, with the patient in a supine position, and is relatively cumbersome to perform. A new anterior segment optical coherence tomography (AS‐OCT) system has recently been introduced which uses a longer wavelength than conventional posterior segment OCT imaging devices (1.3 μm), allowing deeper penetration and cross‐sectional imaging of the anterior chamber and visualisation of the angle.15 AS‐OCT has the benefits of being a rapid, non‐contact method that may be performed by a technician and therefore can be integrated more easily into clinical practice. We compared the changes in angle morphology when going from bright illumination to darkness to those seen after iridotomy using the AS‐OCT as we have done in previous work with the UBM.13
Materials and methods
Subjects seen consecutively at the glaucoma clinic at the National University Hospital (NUH) of Singapore who were diagnosed on gonioscopy to have suspected primary angle closure (as defined by appositional contact between the peripheral iris and posterior trabecular meshwork, in the absence of raised IOP or peripheral anterior synechiae), or to have primary angle closure (with evidence of raised IOP, iris whorling, glaucomflecken and/or excessive pigment deposition on the trabecular surface) or primary angle closure glaucoma (with evidence of glaucomatous optic neuropathy)16 and who were recommended to have LI were invited to participate in this study. People with peripheral anterior synechiae were excluded. Study protocols were reviewed by the institutional review board at NUH, and written consent was obtained from all participants. The work was carried out in accordance with the World Medical Association's Declaration of Helsinki.
Anterior segment optical coherence tomography
All subjects underwent imaging with a prototype of the AS‐OCT (Carl Zeiss Meditec, Dublin, California) prior to any procedures that involved contact with the eye and at least 1 week after recruitment. Details of AS‐OCT imaging technology and methods used here have been previously described;17 the prototype used here provided an image size of 16 mm length‐wise and 8 mm depth‐wise. With the subject in the sitting position and fixating on an internal target, images were captured of the inferior (6 o'clock meridian), nasal and temporal angle quadrants (3 and 9 o'clock meridians). Due to the design of the prototype (a bar used as a rest for the forehead makes it difficult for subjects to pull their upper eyelids upwards and away from their corneal limbus) it was not possible to obtain images of the superior angle. (The commercial version of the AS‐OCT (Visante) has a forehead rest that is much reduced in size and is hinged from only one side, therefore allowing the superior angle to be imaged more easily.) Imaging was first performed in dark conditions (20 lux) and then under bright conditions (4800 lux) after waiting for a minimum of 5 minutes between measurements using a standardised light source. Three images were taken for each quadrant in dark and light conditions. A single observer (JS) performed imaging on all subjects prior to gonioscopic examination. The AS‐OCT images were processed later using custom software (Mathworks Inc., Natick, MA) that corrected for image distortions arising from the refractive effect of the cornea and aqueous (see below).
Slit‐lamp and other examination
Subjects underwent slit‐lamp examination including gonioscopy. Both ultrasound (Sonomed Model A2500 with a 20 MHz A‐mode hard‐tipped probe) and optical (IOL Master, Model 07740 JENA, Carl Zeiss) measurements of the anterior chamber depth were made. The latter were used for analysis as we believe these to be more reliable and accurate.18
Gonioscopy
Subjects underwent gonioscopy by a second, independent observer (WN) with extensive experience in performing gonioscopy in a research setting, who was masked to AS‐OCT findings. All subjects were examined in a totally darkened room with a Goldmann two‐mirror lens. A 1 mm beam of light was reduced to a very narrow slit. The vertical beam was offset horizontally for assessing superior and inferior angles, and a vertically offset horizontal beam used for nasal and temporal angles. Care was taken to avoid light falling on the pupil during gonioscopy. The assessment was carried out at x16 magnification. All four quadrants were assessed with the eye in the primary position of gaze. Minimal adjustment of the lens or eye was allowed to enable an “over the hill” view of a steep iris while avoiding excessive eye movement and manipulation so as to gain an undistorted view of the angle. A Volk four‐mirror indentation goniolens was used where necessary to determine if angle closure was due to apposition or peripheral anterior synechiae. Angle width for all four quadrants was graded as follows: the width in degrees was estimated between the posterior pigmented trabecular meshwork and the peripheral one‐third of the iris (Spaeth grading system). The structures identified were also recorded.
Laser iridotomy
Sequential Argon/YAG laser peripheral iridotomies (LI) were performed using the following settings: Argon (0.7–1.0 W, spot size 50 μm, duration 0.1 second, 10–30 burns) followed by Nd‐YAG (2–5 mJ, 3–5 shots). All subjects received topical prednisolone acetate 1% four times a day for 10 days. Post‐LI evaluations were carried out 2 weeks after the procedure. All iridotomies were confirmed to be patent and of adequate size. One subject was on systemic amitriptyline (an antidepressant with anticholinergic activity) during the study period.
Data analysis
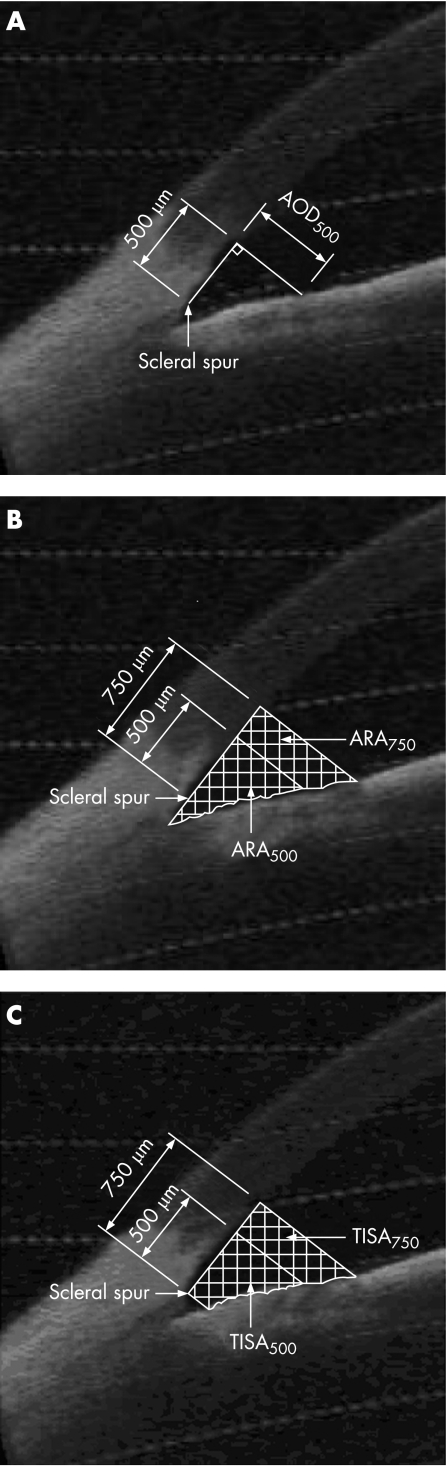
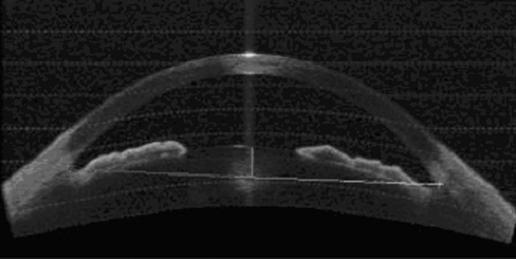
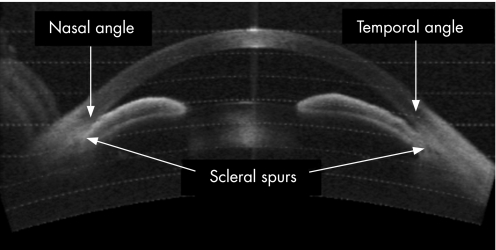
One of the three images per quadrant was selected for analysis based on quality and visibility of the angle structures. Only images with clearly discernible scleral spurs were included in this study. These images were then analysed using custom software (Mathworks Inc., Natick, MA) and standardised angle variables (defined by Pavlin and Ishikawa14,19,20,21) including angle opening distance at 500 microns anterior to the scleral spur (AOD500),19 and a modification of the angle recess area (ARA),20 the trabecular‐iris space area (TISA)17 were calculated after manually marking the scleral spur (fig 1A,B,C). In addition, the central corneal thickness was measured on the image using the measuring caliper function and the lens position in the anterior chamber was calculated by drawing a line from scleral spur to scleral spur and calculating the length of the perpendicular from this to the centre and most anterior point of the anterior surface of the lens (fig 2). Finally, we calculated the angle illumination amplitude (AIA) which was defined as the change in angle biometric variable when going from bright illumination to darkness. We also calculated the log ratio of the change with illumination before and after LI. In cases where the angle was wider in illumination, we set the difference to 0.0001 assuming that the outcome was due to test variability and not to true angle widening. Figure 3 shows an AS‐OCT image of a patient with closed angles and scleral spurs used as landmarks for image processing.
Figure 1 (A) Angle opening distance at 500 μm anterior to the scleral spur (AOD500), defined as the distance from the corneal endothelium to the anterior iris perpendicular to a line drawn along the trabecular meshwork, at 500 μm from the scleral spur. (B) Area of recessed angle at 500 μm or 750 μm from the scleral spur (ARA500, ARA750), defined as the area bounded by the corneal endothelium, trabecular meshwork and anterior iris surface out to a distance of 500 μm or 750 μm from the scleral spur including the lake of fluid posterior to the scleral spur. (C) Trabecular‐iris space area up to 500 μm or 750 μm from the scleral spur (TISA500, TISA750), defined as the area bounded by the corneal endothelium, trabecular meshwork and anterior iris surface out to a distance of 500 μm or 750 μm from the scleral spur, excluding the lake of fluid posterior to the scleral spur.
Figure 2 Lens position in the anterior chamber.
Figure 3 AS‐OCT image showing both nasal and temporal angles closed and scleral spurs used as landmarks.
Comparisons of AS‐OCT findings in the light and dark and before and after LI were performed using paired t tests. Comparisons of the proportion with angle closure before and after LI were performed using McNemar's test. Statistical analyses were performed using SAS version 8.2 (Carey, North Carolina, USA).
Results
Twenty‐seven patients were enrolled consecutively (10 of whom were excluded from this analysis due to the presence of PAS) and seen at two‐week follow‐up. Nine (52.3%) subjects were men, and the mean age of the 17 subjects was 55.8±12.7 years (range 46 to 67 years). There were 16 Chinese subjects (94.1%) and 1 Eurasian subject.
Gonioscopy
Only one of the 17 subjects without PAS was found to have visible pigmented posterior trabecular meshwork in the inferior angle prior to LI, while 15 inferior angles were open after LI. Of the 16 angles closed superiorly on gonioscopy before LI, 13 were open after LI. All 17 temporal angles were open after LI, while 11 had been closed before, as were all 17 nasal angles (7 had been closed before LI). Overall 12 eyes (71%) had three or more quadrants closed before LI while none were classified as having iridotrabecular contact in three or more quadrants after, with 12 eyes (71%) having all four quadrants open after LI.
Changes in angle variables in response to LI
Changes in angle variables after LI were seen both in light and dark (table 1).
Table 1 Ocular biometry using IOL master and AS‐OCT before and after laser iridotomy.
| Parameter | Pre‐LI | Post‐LI | Difference (95% CI) | p |
|---|---|---|---|---|
| a. Measured in the light | ||||
| Mean lens position (mm) | 0.97 | 0.92 | −0.05 (−0.12 to 0.02) | 0.14 |
| Mean ACD (mm) | 2.08 | 2.09 | 0.01 (−0.03 to 0.05) | 0.59 |
| Mean corneal thickness (mm) | 0.59 | 0.57 | −0.01 (−0.03 to 0.003) | 0.10 |
| Mean AOD500 (μm) | 124.4 | 262.5 | 138.1 (112.2 to 164.0) | 0.0001 |
| Mean TISA750 (mm2) | 0.075 | 0.155 | 0.080 (0.061 to 0.10) | 0.0001 |
| b. Measured in the dark | ||||
| Mean lens position (mm) | 0.96 | 0.94 | −0.01 (−0.06 to 0.04) | 0.59 |
| Mean ACD (mm) | 2.08 | 2.10 | 0.01 (−0.01 to 0.04) | 0.21 |
| Mean corneal thickness (mm) | 0.59 | 0.58 | −0.01 (−0.03 to 0.01) | 0.31 |
| Mean AOD500 (μm) | 43.0 | 126.9 | 83.92 (60.3 to 107.6) | 0.0001 |
| Mean TISA750 (mm2) | 0.024 | 0.076 | 0.05 (0.03 to 0.07) | 0.0001 |
ACD, anterior chamber depth; AOD500, angle opening distance at 500 μm anterior to the scleral spur; TISA750, trabecular‐iris space area up to 750 μm from the scleral spur.
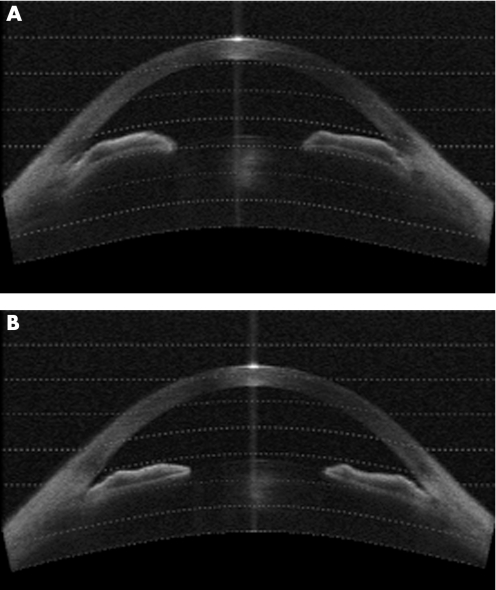
Setting a TISA500 of zero as “closed”, 6 eyes had two or more quadrants satisfying this criterion (35%) before LI, while only one persisted in appearing this closed on OCT after LI (p<0.05). The ACD, lens position and central corneal thickness did not change significantly after LI (p⩾0.1 in both the light and the dark). The AOD500 in the light nearly doubled from 124.4 μm to 262.5 μm, with a similar near doubling of the TISA750 (p<0.001 for both). Figure 4 illustrates a patient with occludable angles which open after LI.
Figure 4 (A) AS‐OCT image showing angle closure before laser peripheral iridotomy. (B) AS‐OCT image showing the same angles open after laser peripheral iridotomy.
Changes in anterior chamber angle variables in response to illumination
There was no statistically significant difference in ACD, lens position or CCT in response to increased illumination (p>0.20). Considering the mean values of temporal, nasal and inferior quadrants, mean AOD500 and mean TISA750 were both nearly three times greater in light than in dark before a LI had been performed (table 2).
Table 2 Dark–light changes in ocular biometry using IOL master and AS‐OCT prior to laser iridotomy.
| Parameter | Dark | Light | Difference (95% CI) | p |
|---|---|---|---|---|
| Mean lens position (mm) | 0.96 | 0.97 | 0.01 (−0.06 to 0.09) | 0.70 |
| Mean ACD (mm) | 2.08 | 2.08 | 0 (−0.03 to 0.0.43) | 0.86 |
| Mean corneal thickness (mm) | 0.59 | 0.59 | 0 (−0.01 to 0.01) | 1.00 |
| Mean AOD500 (μm) | 43.0 | 124.4 | 81.4 (46.0 to 116.8) | <0.001 |
| Mean TISA750 (mm2) | 0.024 | 0.075 | 0.051 (0.03 to 0.07) | <0.001 |
ACD, anterior chamber depth; AOD500, angle opening distance at 500 μm anterior to the scleral spur; TISA750, trabecular‐iris space area up to 750 μm from the scleral spur.
These findings were consistent in all three quadrants studied by AS‐OCT. The inferior angle was the narrowest and the temporal angle was the widest in both light and dark (p<0.01, p = 0.08). The change in angle width going from light to dark (angle illumination amplitude [AIA]) was greater after LI than before. The AIA for AOD500 after LI was on average 54.1 μm greater (baseline before iridotomy = 81.4 μm, p<0.001), the AIA for the mean TISA500 was 0.016 mm2 greater (baseline = 0.38 mm2, p = 0.03) and the AIA for TISA750 was on average 0.028 mm2 greater (baseline = 0.051 mm2, p = 0.02). However, the mean changes in all three variables were largely attributable to a greater AIA in the inferior quadrant after LI. There were similar statistically significant changes in the proportionate decrease in AOD500, TISA500 and TISA750 when going from light to dark (p<0.05 for all three).
Discussion
This study confirms previous reports of increased anterior chamber angle opening in response to illumination and after LI in Asian eyes.13 There was a corresponding increase in gonioscopic opening and a decrease in the number of angles graded as occludable. The AS‐OCT was therefore able to identify clinically important changes in anterior chamber angle morphology with results comparable to those obtained with the UBM (data not presented). The AS‐OCT is easier to use than UBM, however, and requires no instillation of medication or manipulation of the eye.
Several authors have documented with UBM that iridotomy leads to angle widening. Gazzard et al. reported in a study of 55 eyes in a similar Asian population that AOD250, AOD500 and ARA all significantly increased after LI (p ⩽ 0.001) and that gonioscopic grading of the angle opening significantly increased in all four quadrants (p<0.001).13 Using three quadrants with iridotrabecular contact to define a closed angle, the number of eyes classified as closed decreased from 73% to 33%. The present study reported a similar number of angles at baseline as being closed (71%), with none having three closed angles after LI, but the populations studied were not the same. The Gazzard paper reported on contralateral eyes of persons with acute attacks, and the current report is on all persons felt to need a LI without PAS who were identified in a clinic.
Consistent with the theory that the entire iris falls back with iridotomy is the finding of Caronia and colleagues who reported an increase in lens–iris contact in 13 white and Hispanic patients after LI for appositional closure without PAS.22 Marraffa et al. looked at the effects of LI in 30 Italian subjects with PACG and reported that the irido‐trabecular angle nearly doubled from 10.69±8.88° to 21.03±11.28° (p<0.001).23 Our findings using AS‐OCT are similar, with a near doubling of both AOD500 and TISA750 after LI.
In addition, we found that the absolute amount of angle opening in response to illumination (AIA) was greater after LI than before. This may in part be due to the wider angle width (AOD500 and TISA750 were nearly twice as wide after LI with the lights on, so there was a greater ability of the eye to shallow). However, if we look at this in terms of proportionate change in the amount of angle opening, there was still an increase in the angle response after LI, indicating that the dynamic reaction of the eye to illumination may in fact be altered by LI. These changes may also suggest greater mobility of the iris after LI due to decreased iris tension after resolution of pupil block.
This study has certain limitations. Image processing using the current analysis software requires the operator to accurately define the position of the scleral spur on the image before the various variables can be calculated. This step introduces some variability. AS‐OCT image processing would almost certainly be more reproducible using automated techniques as has been done for the UBM.25 The amount of inter‐operator and intra‐operator variability in the acquisition of AS‐OCT images is the subject of a separate paper.26 The person analysing the images was masked to the status of the eye; therefore bias is unlikely to have contributed to the findings on AS‐OCT. However, complete masking during gonioscopy was not possible. While we attempted to perform gonioscopy without assessing for the presence or absence of a patent LI, it is difficult to avoid seeing the LI through the gonioscopy lens. Even with these limitations, our findings of angle widening on gonioscopy are consistent with the AS‐OCT findings and with the findings of previous researchers.13,27
In summary, we have demonstrated that both LI and increased illumination can significantly widen the angle and that AS‐OCT is capable of quantifying this similarly to UBM. Furthermore, the angle closes more in response to illumination both in absolute and proportionate terms after LI. Given the ability of AS‐OCT to detect angle changes in response to stimuli, it may be a useful tool in assessing angle morphology in certain populations both at baseline and over time.
Acknowledgements
The authors would like to thank Dr Yan Li of the Doheny Eye Institute for the use of analysis software in this study and Dr Sunita Radhakrishnan for her assistance.
Contribution of Authors
Design and conduct (JS, PC, SS, WN, PF, TA, DF); collection, management, analysis and interpretation of the data (JS, PC, SS, WN, PF, TA, DF, YHC, CZ), preparation, review or approval of the manuscript (JS, PC, SS, WN, PF, TA, DF, YHC, CZ, DH).
Abbreviations
AOD - angle opening distance
AS‐OCT - anterior segment optical coherence tomography
IOP - intraocular pressure
LI - laser iridotomy
PACG - primary angle‐closure glaucoma
UBM - ultrasound biomicroscopy
Footnotes
Funding: Carl Zeiss Meditec loaned the anterior segment OCT for the study and provided technical support.
SDS and TA have received financial support for travel to conferences from Carl Zeiss Meditec. DSF has previously been a consultant to Carl Zeiss Meditec. DH receives patent royalty and research grant from Carl Zeiss Meditec.
References
- 1.Erie J C, Hodge D O, Gray D T. The incidence of primary angle‐closure glaucoma in Olmstead County, Minnesota. Arch Ophthalmol 1997115177–181. [DOI] [PubMed] [Google Scholar]
- 2.Foster P J. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001851277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandona L, Dandona R, Mandal P.et al Angle‐closure glaucoma in an urban population in southern India. The Andhra Pradesh eye disease study. Ophthalmology 20001071710–1716. [DOI] [PubMed] [Google Scholar]
- 4.Foster P J, Baasanhu J, Alsbirk P H.et al Glaucoma in Mongolia: A population‐based survey in Hövsgöl Province, Northern Mongolia. Arch Ophthalmol 19961141235–1241. [DOI] [PubMed] [Google Scholar]
- 5.Foster P J, Oen F T, Machin D.et al The prevalence of glaucoma in Chinese residents of Singapore: a cross‐sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 20001181105–1111. [DOI] [PubMed] [Google Scholar]
- 6.Seah S K L, Foster P J, Chew P T.et al Incidence of acute primary angle‐closure glaucoma in Singapore. An island‐wide survey. Arch Ophthalmol 19971151436–1440. [DOI] [PubMed] [Google Scholar]
- 7.Quigley H A. Number of people with glaucoma worldwide. Br J Ophthalmol 199680389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anonymous Laser peripheral iridotomy for pupillary‐block glaucoma. American Academy of Ophthalmology. Ophthalmology 19941011749–1758. [DOI] [PubMed] [Google Scholar]
- 9.Fleck B W, Dhillon B, Khanna V.et al A randomised, prospective comparison of Nd:YAG laser iridotomy and operative peripheral iridectomy in fellow eyes. Eye 19915315–321. [DOI] [PubMed] [Google Scholar]
- 10.Saunders D C. Acute closed‐angle glaucoma and Nd‐YAG laser iridotomy. Br J Ophthalmol 199074523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan W P, Foster P J, Devereux J G.et al YAG laser iridotomy treatment for primary angle closure in east Asian eyes. BJO 2000841255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGalliard J N, Wishart P K. The effect of Nd:YAG iridotomy on intraocular pressure in hypertensive eyes with shallow anterior chambers. Eye 19904823–829. [DOI] [PubMed] [Google Scholar]
- 13.Gazzard G, Friedman D, Devereux J.et al A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology after laser iridotomy in Asian eyes. Ophthalmology 2003110630–638. [DOI] [PubMed] [Google Scholar]
- 14.Pavlin C J, Foster F S. Ultrasound biomicroscopy in glaucoma. Acta Ophthalmologica 1992(Suppl 204)7–9. [DOI] [PubMed]
- 15.Radhakrishnan S, Rollins A M, Roth J E.et al Real‐time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 20011191179–1185. [DOI] [PubMed] [Google Scholar]
- 16.Foster P J, Buhrmann R, Quigley H A.et al The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 200286238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishnan S, Goldsmith J, Westphal V.et al Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 20051281053–1059. [DOI] [PubMed] [Google Scholar]
- 18.Foster P J, Alsbirk P H, Baasanhu J.et al Anterior chamber depth in Mongolians. Variation with age, sex and method of measurement. Am J Ophthalmol 199712453–60. [DOI] [PubMed] [Google Scholar]
- 19.Pavlin C J, Harasiewicz K, Foster F S. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol 1992113381–389. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa H, Liebmann J, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol 200011133–139. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa H, Esaki K, Liebmann J M.et al Ultrasound biomicroscopy dark room provocative testing: a quantitative method for estimating anterior chamber angle width. Jpn J Ophthalmol 199943526–534. [DOI] [PubMed] [Google Scholar]
- 22.Caronia R M, Liebmann J M, Stegman Z.et al Increase in iris‐lens contact after laser iridotomy for pupillary block angle closure. Am J Ophthalmol 199612253–57. [DOI] [PubMed] [Google Scholar]
- 23.Marraffa M, Marchini G, Pagliarusco A.et al Ultrasound biomicroscopy and corneal endothelium in Nd:YAG‐laser iridotomy. Ophthalmic Surgery & Lasers 199526519–523. [PubMed] [Google Scholar]
- 24.Landers J, Craig J. Decompression retinopathy and corneal oedema following Nd:YAG laser peripheral iridotomy. Clin Experiment Ophthalmol 200634182–184. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa H, Liebmann J M, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol 200011133–139. [DOI] [PubMed] [Google Scholar]
- 26.Radhakrishnan S, See J, Smith S D.et al Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. IOVS Manuscript #06‐1120 (accepted for publication) [DOI] [PubMed]
- 27.Lim L S, Aung T, Husain R. Acute primary angle closure: configuration of the drainage angle in the first year after laser peripheral iridotomy. Ophthalmol 20041111470–1474. [DOI] [PubMed] [Google Scholar]