Abstract
Aims
To investigate the presence and prognostic relevance of KIT expression in paediatric renal tumours, and to determine whether receptor overexpression is associated with gene amplification and/or mutation.
Methods
Immunohistochemistry without antigen retrieval for CD117 was carried out on tissue microarrays consisting of 274 Wilms' tumours, 13 clear cell sarcomas of the kidney (CCSK), 10 mesoblastic nephromas (MN), and 7 rhabdoid tumours of the kidney (RTK). In addition, gene copy number was investigated by chromogenic in situ hybridisation (CISH), and overexpressing tumours were sequenced for KIT mutations in exons 9, 11, 13 and 17.
Results
Only 8/200 (4.0%) Wilms' tumours exhibited any degree of moderate–strong KIT staining in any of their assessable cell types. This small group of KIT‐positive tumours had a shorter time to relapse (p = 0.0044, log‐rank test). There were no positive MNs or RTKs; however 3/11 (27.3%) CCSKs were strongly positive, with an additional two cases weakly reactive. No cases exhibited gene amplification or mutation.
Conclusions
KIT overexpression in rare in Wilms' tumours, although does appear to confer a worse prognosis, in particular for patients primarily treated with preoperative chemotherapy. CCSKs are associated with an increased expression of KIT, however, in the absence of gene amplification and/or activating mutation. The potential of anti‐KIT therapeutic strategies in the treatment of paediatric renal tumours appears to be limited.
The proto‐oncogene c‐kit (KIT, CD117) encodes a type III receptor tyrosine kinase which, on binding of the ligand SCF (stem cell factor), transduces signals important in a variety of normal physiological and pathogenic processes, including cell survival and proliferation, migration, and differentiation.1 Response to targeted therapy by imatinib mesylate (STI‐571, Gleevec) in KIT‐overexpressing malignancies such as gastrointestinal stromal tumours (GISTs)2 has driven the desire to identify other tumour types which may also be candidates for such a therapeutic approach.
The KIT receptor is normally expressed in mast cells, melanocytes and basal cells of the skin, breast epithelium, some haematopoietic stem cells, mast cells, germ cells and Cajal cells of the gastrointestinal tract.3 KIT is not expressed in the normal squamous epithelium or the glandular epithelium of the endocervix, prostate, stomach, intestine and pancreas.4 In addition to GISTs, other tumours exhibiting a percentage of cases with KIT positivity include adenoid cystic carcinomas, angiosarcomas, Ewing sarcomas, testicular germ cell tumours, small cell lung adenocarcinomas and melanomas.5,6 Despite this wide‐ranging pattern of overexpression, KIT mutations are uncommon in tumours other than GISTs.7
In the kidney, KIT is reported to be expressed at moderate levels in the epithelial cells of the proximal and distal tubules, with renal corpuscles, loops of Henle and collecting tubules negative for the receptor.8 Expression was also noted in the developing proximal tubules, and to a lesser extent the distal tubules of fetal kidney. In the few previous studies examining renal tumours, most conventional renal cell carcinomas (RCC) were found to be KIT negative, although a subset of clear cell, papillary type and chromophobe RCCs, all oncocytomas, and most mesoblastic nephromas were reported to be immunoreactive for antibodies directed against CD117 antigen.8 In the paediatric setting, 0/68 and 1/219 Wilms' tumours (nephroblastoma) were found to be KIT positive by immunohistochemistry, suggesting a minimal importance in these neoplasms. In a recent gene expression profiling study, KIT mRNA was found to be differentially expressed in clear cell sarcomas of the kidney (CCSK) compared with fetal kidney and Wilms' tumour samples, providing the first evidence for a potential therapeutic application for KIT‐directed compounds in a childhood kidney cancer.10
In this study we aimed to evaluate KIT receptor expression, DNA copy number and mutational status in a wide range of paediatric renal tumours in order to correlate with clinicopathological parameters and evaluate the potential for novel targeted therapies in these patients. Our finding of rare, but prognostically important KIT‐positivity in Wilms' tumours, as well as significantly raised levels in CCSKs highlights the possibility of a role for KIT in poor outcome in these malignancies; however the absence of gene amplification and mutation suggests that the use of imatinib may not be warranted.
Materials and methods
Tissue microarrays
Paediatric renal tumour tissue microarrays were constructed11 containing replicate representative cores (n = 885) from all available cellular components from 274 Wilms' tumours, 13 clear cell sarcomas of the kidney (CCSK), 10 mesoblastic nephromas (MN; 7 classic and 3 cellular), and 7 rhabdoid tumours of the kidney (RTK). Tumours were treated by either immediate nephrectomy or delayed nephrectomy following preoperative chemotherapy. There was a slight enrichment of tumours which relapsed (19.5% Wilms' tumours) due to the presence of consultation cases. The presence of tumour tissue on the arrayed samples was verified on an H&E stained section. Tumour cell positivity and cellular distribution were assessed independently by three pathologists (MR‐P, JSR‐F, GV).
Immunohistochemistry
Immunohistochemistry for KIT was performed on 4 μm formalin‐fixed, paraffin‐embedded (FFPE) sections using a primary antibody raised against CD117 (clone A4502, Dako, Glostrup, Denmark), using the Envision–HRP system (K4006, Dako) at a dilution of 1:50, as previously described.12 No antigen retrieval was used.13 Only membranous KIT immunoreactivity with or without cytoplasmic staining in the tumour cells was considered positive. Both tissue microarray and whole sections were semi‐quantitatively analysed. The intensity of the reaction was scored as negative, weak, or strong, and in the whole sections the distribution was additionally assessed. Only strong reactivity in >10% cells was considered definitively “strongly positive”.
Chromogenic in situ hybridisation
Chromogenic in situ hybridisation (CISH) was carried out using 500 ng of GenomiPhi‐amplified BAC DNA (RP11‐42B10, RP11‐586A02 and RP11‐273B19), FISH‐mapped and end‐sequenced, localising to 4q12 (∼55.0 Mb–55.5 Mb) according to the March 2006 human genome assembly (hg18). Probes were labelled with biotin (Bioprime labelling kit, Invitrogen, Paisley, UK) and hybridised as previously described.12,14 Deparaffinised sections were incubated for 15 min at 98°C in CISH pretreatment buffer (SPOT‐light tissue pretreatment kit, Zymed, San Francisco, CA, USA) and digested with pepsin for 6 min at room temperature. CISH experiments were analysed independently by three pathologists (MR‐P, JSR‐F, GV). Sixty morphologically unequivocal neoplastic cells were counted for the presence of KIT probe signals. Amplification was defined as >5 signals per nucleus in more than 50% of the tumour cells, or when large gene copy clusters were seen.12,14,15
Mutation analysis
Genomic DNA was isolated from 10 μm thick unstained tissue sections containing >85% tumour cells, as determined from a serial H&E stained section, using the QiaAmp DNA mini kit (Qiagen, Crawley, UK) according to the manufacturer's instructions. Exons 9, 11, 13 and 17 of the KIT gene were screened for mutations by bidirectional direct sequencing as previously described.12 The primer sequences for the four exons are as follows: exon 9 (forward) ATG CTC TGC TTC TGT ACT GCC, (reverse) CAG AGC CTA AAC ATC CCC TTA; exon 11 (forward) CCA GAG TGC TCT AAT GAC TG, (reverse) ACC CAA AAA GGT GAC ATG GA; exon 13 (forward) CAT CAG TTT GCC AGT TGT GC, (reverse) ACA CGG CTT TAC CTC CAA TG; exon 17 (forward) TGT ATT CAC AGA GAC TTG GC, (reverse) GAA ACT AAA AAT CCT TTG CAG GAC. Only those cases with strong protein overexpression observed by immunohistochemistry were screened for activating mutations. Sequencing reactions were carried out in duplicate.
Statistical analysis
All statistical tests were performed in R2.3 (http://www.r‐project.org/). Correlations between categorical values were performed using the χ2 and Fisher's exact tests. Correlations between continuous and categorical variables were performed using analysis of variance, when continuous values showed a normal distribution. Cumulative survival probabilities were calculated using the Kaplan–Meier method, with differences between survival rates analysed with the log‐rank test. Multivariate analysis was carried out using the Cox proportional hazards model. All tests were two‐tailed, with a confidence interval of 95%.
Results
Immunohistochemistry for KIT receptor was carried out on a paediatric renal tumour tissue microarray without antigen retrieval and scored rigorously to exclude false positives. In all, 226 tumours were assessable by this approach, with the results summarised in table 1.
Table 1 Summary of KIT expression data for immunohistochemistry on a paediatric renal tumour tissue microarray.
| Histology | KIT immunohistochemistry | ||
|---|---|---|---|
| Strongly positive | Weakly positive | Negative | |
| FH WT | 8 (4.2%) | 25 (13.2%) | 156 (82.5%) |
| Anaplastic WT | 0 | 2 (18.2%) | 9 (81.8%) |
| CCSK | 3 (27.3%)* | 2 (18.2%) | 6 (54.5%) |
| MN | 0 | 0 | 7 (100%) |
| RTK | 0 | 1 (16.7%) | 5 (83.3%) |
| PNET | 0 | 0 | 1 (100%) |
| RCC | 0 | 0 | 1 (100%) |
A significantly increased proportion of CCSKs were positive compared to favourable histology Wilms' tumours (*p = 0.0206, Fisher's exact test).
WT, Wilms' tumour; FH, favourable histology; CCSK, clear cell sarcoma of the kidney; MN, mesoblastic nephroma; RTK, rhabdoid tumour of the kidney; PNET, primitive neuroectodermal tumour; RCC, renal cell carcinoma.
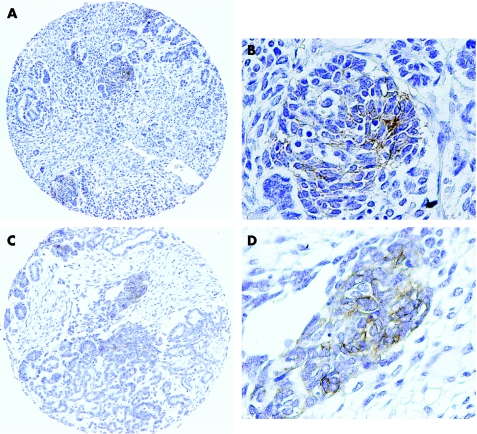
In favourable histology Wilms' tumour, which formed the vast majority of cases on the tissue array, blastemal cells showed a strong membranous/cytoplasmic reactivity in only 5/107 (4.7%) assessable tumours. Thirteen cases showed a weak reactivity. This was largely mirrored when the epithelial component was assessed, with 4/70 (5.7%) tumours unequivocally positive, and 7 cases showing only weak staining. Only a single case showed strong reactivity in both compartments. There were no cases with a strongly positive tumourigenic stroma, although 7/100 showed a weak reactivity. Taken together, the percentage of Wilms' tumours which exhibited any degree of strong staining in any of their assessable cell types was 8/189 (4.2%). No strong immunoreactivity was observed in any anaplastic Wilms' tumours, although 2/11 (18.2%) exhibited weak staining. Figure 1 shows examples of the staining observed.
Figure 1 Expression of KIT receptor in Wilms' tumours. Representative examples of KIT‐positive Wilms' tumours from the paediatric renal tumour tissue microarray. (A) Low power view of case RMH1955, showing focal positivity in the epithelial cells. Original magnification ×200. (B) High power view of RMH1955. Original magnification ×400. (C) Low power view of case RMH1347, showing focal positivity in the blastemal cells. Original magnification ×200. (D) High power view of RMH1347. Original magnification ×400.
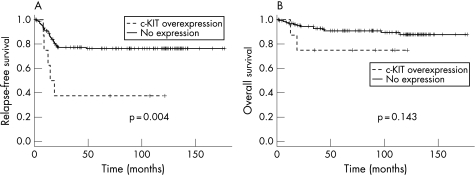
Across all Wilms' tumours, there were no correlations between KIT positivity and tumour stage (p = 0.36, Fisher's exact test) or age (p = 0.74, Fisher's exact test for age at diagnosis of less than or greater than 24 months). Taking those positive favourable histology Wilms' tumours which were strongly positive versus the remaining cases, KIT immunoreactivity showed a significant association with a shorter time to relapse (p = 0.0044, log‐rank test). There was no such association with overall survival (p = 0.14, log‐rank test, fig 2). This association was driven by the strong prognostic relevance of KIT positivity of those tumours treated by preoperative chemotherapy (p = 0.0002, log‐rank test for relapse‐free survival), particularly those with expression in the epithelial component (p = 0.031, log‐rank test for relapse‐free survival). When stratified by treatment protocol, no such prognostic significance was observed for those patients who underwent immediate nephrectomy (p = 0.21, log‐rank test for relapse‐free survival). Table 2 summarises the results. Multivariate analysis incorporating stage and age as co‐variables revealed that KIT expression was not an independent prognostic factor for relapse‐free (p = 0.79, Cox proportional hazards model) or overall survival (p = 0.55, Cox proportional hazards model).
Figure 2 Survival analysis for favourable histology Wilms' tumours stratified by KIT expression. Kaplan–Meier curves for (A) relapse‐free and (B) overall survival of favourable histology Wilms' tumours from the tissue microarray. p Values are calculated by the log‐rank test.
Table 2 Clinical correlations for KIT expression in favourable histology Wilms' tumours.
| Cell type | KIT immunohistochemistry | Immediate nephrectomy | Preoperative chemotherapy | Any | |||||
|---|---|---|---|---|---|---|---|---|---|
| Strongly positive | Weakly positive | Negative | RFS | OS | RFS | OS | RFS | OS | |
| WT blastemal | 5 (4.7%) | 13 (12.1%) | 89 (83.2%) | p = 0.69 | p = 0.52 | p = 0.08 | p = 0.16 | p = 0.14 | p = 0.67 |
| WT epithelial | 4 (5.7%) | 7 (10.0%) | 59 (71.4%) | p = 0.29 | NA | p = 0.031* | NA | 0.085 | NA |
| WT stromal | 0 | 7 (7.0%) | 93 (93.0%) | NA | NA | NA | NA | NA | NA |
| WT any | 8 (4.2%) | 25 (13.2%) | 156 (82.5%) | p = 0.21 | p = 0.42 | p = 0.0002* | p = 0.19 | p = 0.0044* | p = 0.45 |
Wilms' tumour cases from the tissue microarray are stratified according to cellular component. p Values are calculated for relapse‐free (RFS) and overall (OS) survival, stratified by treatment type.
*Significant associations.
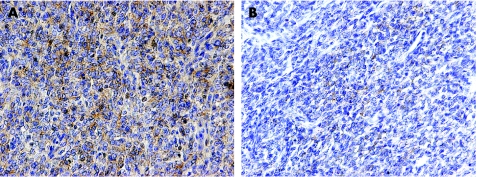
Focusing on other paediatric renal tumours, no moderate–strong positivity was observed in any of seven mesoblastic nephromas, or six rhabdoid tumours of the kidney (although one case showed weak staining). Similarly, a single case each of primitive neuroectodermal tumour and RCC was negative. In contrast, of assessable CCSKs, 3/11 (27.3%) were strongly positive, a significantly increased percentage compared with Wilms' tumour (p = 0.016, Fisher's exact test). An additional two CCSK cases were weakly reactive. In order to investigate these cases further, immunohistochemistry for all CCSKs was repeated on whole sections. There was good agreement with the tissue array data, with the same 3/11 assessable tumours unequivocally positive (fig 3). Table 3 presents the data for each CCSK case.
Figure 3 KIT expression in clear cell sarcoma of the kidney. Examples of overexpression of KIT in whole sections of CCSK. (A) Strong expression in RMH0753. Original magnification ×200. (B) Weak expression in RMH1931. Original magnification ×200.
Table 3 Summary of clear cell sarcoma of the kidney (CCSK) cases.
| Case no. | Diagnosis | Treatment | Age (months) | Stage | KIT IHC | KIT CISH | KIT mutation |
|---|---|---|---|---|---|---|---|
| RMH0471 | CCSK | Preoperative chemotherapy | NA | NA | Strong, 10–33% | NA | Negative |
| RMH0751 | CCSK | Immediate nephrectomy | 19 | 2 | Strong, 10–33% | Not amplified | Negative |
| RMH0753 | CCSK | Immediate nephrectomy | 20 | 2 | Strong, >70% | Not amplified | Negative |
| RMH1226 | CCSK | Preoperative chemotherapy | 22 | 3 | Negative | Not amplified | NA |
| RMH1278 | CCSK | Preoperative chemotherapy | 28 | 3 | Negative | Not amplified | NA |
| RMH1286 | CCSK | Preoperative chemotherapy | 12 | 3 | Negative | Not amplified | NA |
| RMH1330 | CCSK | Preoperative chemotherapy | 28 | 3 | Negative | NA | NA |
| RMH1518 | CCSK | Preoperative chemotherapy | 7 | 3 | Negative | Not amplified | NA |
| RMH1826 | CCSK | Preoperative chemotherapy | 24 | 2 | Negative | Not amplified | NA |
| RMH1880 | CCSK | Immediate nephrectomy | NA | NA | Negative | Not amplified | NA |
| RMH1910 | CCSK | Immediate nephrectomy | 15 | NA | Weak, 10–33% | Not amplified | NA |
| RMH1931 | CCSK | Immediate nephrectomy | 14 | NA | Weak, 33–66% | Not amplified | NA |
NA, not applicable.
No gene amplification or activating mutations were observed.
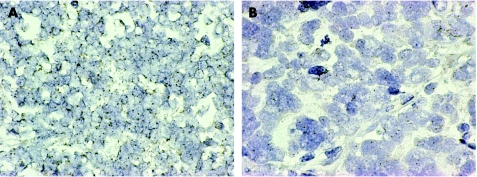
In order to determine whether an increased gene copy number of KIT may be associated with receptor overexpression, CISH was performed on the tissue array, as well as whole sections of the cases with a strong immunohistochemical reactivity. In none of the assessable cases was increased DNA copy number indicative of gene amplification observed (>5 signals per nucleus in more than 50% of the tumour cells, or when large gene copy clusters were seen). Only normal copy numbers were seen, as shown in fig 4.
Figure 4 Chromogenic in situ hybridisation of probes directed against KIT in Wilms' tumour and CCSK. No evidence of gene amplification was observed in any KIT overexpressing case. (A) Wilms' tumour RMH1955 and (B) CCSK RMH1931 exhibiting two copies of KIT per nucleus. Original magnification ×630.
Furthermore, we wished to investigate whether activating mutations in KIT may be present in our paediatric renal tumour cohort. All cases with strong overexpression of the protein were analysed for mutations in exons 9, 11, 13 and 17 by direct sequencing. No mutations were observed in either the Wilms' tumours or CCSKs (table 3).
Discussion
The proto‐oncogene KIT has received widespread attention due to the development of effective novel inhibitory therapeutics such as imatinib, and the successful treatment of tumours such as GISTs which are known to overexpress the receptor in >95% of cases. Numerous studies investigating multiple other tumour types which may also benefit from anti‐KIT strategies have since been published looking for other KIT‐positive malignancies. The many inconsistencies that have been observed across such investigations have been suggested to be reflective of technical issues, including choice of primary antibody and antigen retrieval.13 In the present study we have utilised the reported optimal immunostaining procedure12,16 and no antigen retrieval to investigate paediatric renal tumours, and have identified a significant proportion (3/11, 27.3%) of CCSKs to overexpress the receptor.
Although there has been no previous study focusing on KIT expression in paediatric renal tumours, the overexpression of KIT in CCSKs was first identified in a recent oligonucleotide microarray expression profiling experiment examining 15 Wilms' tumours, 14 CCSKs and 3 fetal kidney samples.10 This study identified a distinctive expression profile of CCSKs which comprised a variety of neuronal markers, members of the Sonic hedgehog pathway, members of the PI3Kinase/Akt cell proliferation pathway, as well as known therapeutic targets including KIT.10 Our findings of differential KIT receptor expression in CCSKs compared with other paediatric renal tumours including Wilms' confirms this association.
CCSK is a malignant mesenchymal neoplasm with a striking predilection to metastasise to bone, and comprises approximately 3–5% of paediatric renal tumours, with an incidence peaking during the second year of life and progressively falling thereafter.17 Survival is significantly decreased in comparison with Wilms' tumour, at approx 70%, with metastases occurring as late as 10 years after initial diagnosis,18 providing the impetus for the early adoption of novel therapeutics with potential efficacy in the disease.
We have identified a small subset of Wilms' tumours with high risk of relapse, who also express the receptor to levels that correlate with inhibitor efficacy in other tumour types, and may also benefit from such a molecularly‐targeted intervention. The only two previous studies of KIT expression in Wilms' tumour reported frequencies of 0/6 and 1/21 respectively, in line with our larger study, although no clinicopathological information was available.8,9 Despite the rarity of the finding (8/201, 4%), identification of novel targets for the treatment of relapsing Wilms' tumour is of clinical importance, as despite salvage regimens, approximately 50% of these patients will die of the disease.19 Although there is little difference in clinical outcome between those patients treated with preoperative chemotherapy versus those with immediate nephrectomy, KIT overexpression only correlated with a poor relapse‐free survival in the group which underwent delayed nephrectomy. Thus it appears from our data that rather than being a bona fide prognostic factor, KIT may be a useful predictor of response to neoadjuvant chemotherapy. It is further worth noting that there was no association between the eight cases of KIT‐positive favourable histology Wilms' tumours identified on our tissue microarray and cases overexpressing additional receptor tyrosine kinases in other studies on this cohort, including insulin‐like growth factor 1 receptor20 and epidermal growth factor receptor (data not shown).
Although much success has been observed in KIT‐overexpressing GISTs with imatinib, the vast majority of these tumours have a gain‐of‐function mutation in the KIT proto‐oncogene. Most mutations occur in exon 11, but mutations may also be found in exon 9 and rarely in exons 13 and 17,21 resulting in ligand‐independent activation of KIT signalling, leading to growth and antiapoptotic signals. It is therefore of clinical relevance that no such mutations were observed in our KIT‐overexpressing CCSKs or Wilms' tumours. In GISTs, activating mutations in KIT are predictive for imatinib response, although wild‐type receptors are also inhibited.21,22 Patients without activating mutations rarely respond to imatinib treatment. The data from the present study support a recent publication reporting that KIT mutations are uncommon in solid tumours besides GISTs, despite frequent protein expression in other histological types.7
In the absence of mutation, KIT kinase expression may be associated with the presence of multiple copies of the wild‐type KIT gene in cancer cells. Examination of our KIT overexpression by chromogenic in situ hybridisation, a strategy successfully employed to identify KIT amplification in glioblastomas,23 further revealed no copy number increase in our paediatric renal tumours, including those receptor overexpressing CCSKs and Wilms' tumours. This fits previous genome‐wide analyses, which identified no amplifications at 4q12 in a series of 76 primary Wilms' tumours by array CGH,24 as well as an earlier chromosomal comparative genomic hybridisation study of 30 CCSKs, where no such event was observed.25
Although the lack of activating mutation and gene amplification may be indicative of a limited efficacy of imatinib in CCSKs and Wilms' tumours, KIT signalling may be activated by other mechanisms such as gene fusion, ligand stimulation cross‐activation by other kinases, and epigenetic means, and therefore the value of imatinib treatment in paediatric renal tumours may yet be determined to have some value despite the absence of KIT mutation.
Take‐home messages
In paediatric renal tumours, KIT expression is predominantly restricted to clear cell sarcomas of the kidney (CCSKs).
KIT receptor is expressed in very few favourable histology Wilms' tumours, although these cases may have a worse prognosis.
KIT overexpression in Wilms' tumours and CCSKs is not accompanied by gene amplification or activating mutations.
Acknowledgements
We would like to thank the Children's Cancer and Leukaemia Group (CCLG) Tumour Bank, which is funded by Cancer Research UK, as well as pathologists, oncologists and surgeons at the contributing CCLG centres for access to samples.
Abbreviations
CCSK - clear cell sarcoma of the kidney
CISH - chromogenic in situ hybridisation
GIST - gastrointestinal stromal tumour
MN - mesoblastic nephroma
RCC - renal cell carcinoma
RTK - rhabdoid tumour of the kidney
Footnotes
Competing interests: None declared.
References
- 1.Ashman L K. The biology of stem cell factor and its receptor C‐kit. Int J Biochem Cell Biol 1999311037–1051. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Roberts P J, Sarlomo‐Rikala M.et al Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 20013441052–1056. [DOI] [PubMed] [Google Scholar]
- 3.Tsuura Y, Hiraki H, Watanabe K.et al Preferential localization of c‐kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: immunohistochemical study on formalin‐fixed, paraffin‐embedded tissues. Virchows Arch 1994424135–141. [DOI] [PubMed] [Google Scholar]
- 4.Lammie A, Drobnjak M, Gerald W.et al Expression of c‐kit and kit ligand proteins in normal human tissues. J Histochem Cytochem 1994421417–1425. [DOI] [PubMed] [Google Scholar]
- 5.Arber D A, Tamayo R, Weiss L M. Paraffin section detection of the c‐kit gene product (CD117) in human tissues: value in the diagnosis of mast cell disorders. Hum Pathol 199829498–504. [DOI] [PubMed] [Google Scholar]
- 6.Natali P G, Nicotra M R, Sures I.et al Expression of c‐kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res 1992526139–6143. [PubMed] [Google Scholar]
- 7.Sihto H, Sarlomo‐Rikala M, Tynninen O.et al KIT and platelet‐derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol 20052349–57. [DOI] [PubMed] [Google Scholar]
- 8.Miliaras D, Karasavvidou F, Papanikolaou A.et al KIT expression in fetal, normal adult, and neoplastic renal tissues. J Clin Pathol 200457463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smithey B E, Pappo A S, Hill D A. C‐kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol 200226486–492. [DOI] [PubMed] [Google Scholar]
- 10.Cutcliffe C, Kersey D, Huang C C.et al Clear cell sarcoma of the kidney: up‐regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin Cancer Res 2005117986–7994. [DOI] [PubMed] [Google Scholar]
- 11.Kononen J, Bubendorf L, Kallioniemi A.et al Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 12.Reis R M, Martins A, Ribeiro S A.et al Molecular characterization of PDGFR‐alpha/PDGF‐A and c‐KIT/SCF in gliosarcomas. Cell Oncol 200527319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornick J L, Fletcher C D. Validating immunohistochemical staining for KIT (CD117). Am J Clin Pathol 2003119325–327. [DOI] [PubMed] [Google Scholar]
- 14.Lambros M B, Simpson P T, Jones C.et al Unlocking pathology archives for molecular genetic studies: a reliable method to generate probes for chromogenic and fluorescent in situ hybridization. Lab Invest 200686398–408. [DOI] [PubMed] [Google Scholar]
- 15.Sauer T, Beraki K, Noren T.et al EGFR gene copy number heterogeneity in fine‐needle aspiration cytology from breast carcinomas determined by chromogenic in situ hybridization. Diagn Cytopathol 200533228–232. [DOI] [PubMed] [Google Scholar]
- 16.Lucas D R, al‐Abbadi M, Tabaczka P.et al c‐Kit expression in desmoid fibromatosis. Comparative immunohistochemical evaluation of two commercial antibodies. Am J Clin Pathol 2003119339–345. [DOI] [PubMed] [Google Scholar]
- 17.Argani P, Perlman E J, Breslow N E.et al Clear cell sarcoma of the kidney: a review of 351 cases from the National Wilms' Tumor Study Group Pathology Center. Am J Surg Pathol 2000244–18. [DOI] [PubMed] [Google Scholar]
- 18.Seibel N L, Li S, Breslow N E.et al Effect of duration of treatment on treatment outcome for patients with clear‐cell sarcoma of the kidney: a report from the National Wilms' Tumor Study Group. J Clin Oncol 200422468–473. [DOI] [PubMed] [Google Scholar]
- 19.Kalapurakal J A, Dome J S, Perlman E J.et al Management of Wilms' tumour: current practice and future goals. Lancet Oncol 2004537–46. [DOI] [PubMed] [Google Scholar]
- 20.Natrajan R, Reis Filho J S, Little S E.et al Blastemal expression of type I insulin‐like growth factor receptor in Wilms' tumors is driven by increased copy number and correlates with relapse. Cancer Res 2006661148–1155. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich M C, Blanke C D, Druker B J.et al Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT‐positive malignancies. J Clin Oncol 2002201692–1703. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich M C, Corless C L, Demetri G D.et al Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003214342–4349. [DOI] [PubMed] [Google Scholar]
- 23.Gomes A L, Reis‐Filho J S, Lopes J M.et al Molecular alterations of KIT oncogene in gliomas. Cell Oncol. In press [DOI] [PMC free article] [PubMed]
- 24.Natrajan R, Williams R D, Hing S N.et al Array CGH profiling of favourable histology Wilms' tumours reveals novel gains and losses associated with relapse. J Pathol 200621049–58. [DOI] [PubMed] [Google Scholar]
- 25.Schuster A E, Schneider D T, Fritsch M K.et al Genetic and genetic expression analyses of clear cell sarcoma of the kidney. Lab Invest 2003831293–1299. [DOI] [PubMed] [Google Scholar]