Abstract
Background
For cancers of the upper gastrointestinal tract it is standard to examine one section/level, from paraffin blocks containing lymph node tissue, for metastatic tumour.
Aims
To determine whether significantly more metastases can be detected by assessing two additional levels.
Methods
101 archival upper gastrointestinal cancers were evaluated. All negative lymph nodes were examined at two additional levels separated by 100 μm and stained by H&E. The slides were examined for the presence of metastases.
Results
1143 lymph nodes, that were originally clear of metastases, were examined at a further two levels (three levels in total); 23 additional metastases were identified in 17 patients. Eleven of these patients were already stage N1 before examination of the additional levels. However, six patients were originally N0, and were therefore upgraded to N1.
Conclusions
Examining lymph nodes at three levels did detect more metastatic deposits than examination of one section/level. In six patients this changed the N stage from N0 to N1. This would have significant prognostic and management implications.
Approximately 15 000 people develop cancer of the oesophagus, oesophagogastric junction and stomach in the UK each year, and in the developing world adenocarcinoma of the gastro‐oesophageal junction is increasing in incidence faster than any other type of gastrointestinal cancer.1,2 Surgical resection remains the mainstay of potentially curative treatment, but is high risk and long‐term survival is disappointing.
The most commonly used staging system in the UK for upper gastrointestinal tumours is the TNM classification system of tumours.3 Lymph node status is one of the most significant, if not the most significant, indicator of prognosis in such cancers.4,5,6,7 The TNM classification places patients with lymph node tumour deposits in category N1 for oesophageal cancer and N1, N2 or N3 for gastric cancer depending on the number of lymph nodes involved. There is no separate TNM classification for tumours of the oesophagogastric junction, and it can be difficult at times for the pathologist to decide which staging classification to use (oesophageal or gastric) for such tumours. There is a lack of evidence based guidance on how best to sample lymph nodes from upper gastrointestinal tumours.
The Royal College of Pathologists' minimum dataset for gastric carcinoma states that lymph nodes identified within the resection specimen should be cut through their greatest diameter and one half taken for microscopy.8 The Royal College of Pathologists' minimum dataset for oesophageal cancer does not comment on how lymph nodes should be sampled; however it does state that there was not enough evidence at the time of publication to support the use of immunohistochemistry and serial sections to detect micrometastases.9 Drafts of the revised datasets for reporting oesophageal and gastric carcinomas are available on the Royal College of Pathologists website.10,11 The revised draft copy of the oesophageal dataset recommends the use of TNM5 over TNM6, but there is still no advice on how to sample lymph nodes. The revised draft copy of the gastric dataset states all lymph nodes found should be sampled, but there is no additional advice on how to do this. In best practice guidelines for handling oesophageal resection specimens, the recommendation for lymph node sampling is to sample lymph nodes clearly replaced by tumour and to completely sample all lymph nodes that appear tumour free.12 The College of American Pathologists recommends evaluating all lymph nodes, but again does not comment on how best to do this.13
On review of the literature, there appears to be little information on the value of serial sections of lymph nodes within oesophageal and gastric carcinomas to detect metastatic carcinoma. In some subspecialty areas, the use of serial sections has been examined. In the area of breast pathology, the National Health Service Breast Screening Programme, published in 1995, did recommend examination of lymph nodes less than 5 mm at two levels.14 However, in the more recent publication (January 2005), examination of levels was stated not to be routinely necessary.15 However, it is recommended that lymph nodes should be sliced at intervals of approximately 3 mm or less, perpendicular to the long axis, as this is an effective and simpler alternative to serial sectioning to detect small metastatic deposits in lymph nodes. The use of triple levelling has been assessed in colorectal carcinoma, and in a study of 100 colorectal carcinoma resection specimens, 12 extra metastases, in 11 patients, were discovered within lymph nodes at levels 2 and 3, which were negative in level 1.16
Despite the lack of information on serial sectioning there have been numerous publications, examining the detection of micrometastases with immunohistochemistry in lymph nodes from resection specimens of the oesophagus and stomach. These studies show an increase in the detection of micrometastases of between 10% and 40%.17,18,19,20,21,22 The detection of micrometastases did not appear to be related to prognosis in the majority of studies examining oesophageal carcinoma, (predominantly squamous in type); however, there did appear to be a reduction in prognosis in patients with micrometastases from gastric adenocarcinomas.
An audit was completed to examine the value of performing three serial sections on lymph nodes from carcinomas of the oesophagus, gastro‐oesophageal junction and stomach to detect increased numbers of metastases.
Methods
A SNOMED search was carried out on telepath to identify cases of oesophagectomy, oesophagogastrectomy, and gastrectomy performed for primary carcinoma between 1995 and 2005. The original reports, slides and paraffin blocks were retrieved from the archives when available and anonymised. The original slides were reviewed and any containing lymph nodes identified. The blocks containing lymph nodes showing no evidence of metastatic tumour had an extra two levels (separated by 100 μm) cut. The original section obtained from the archives was counted as the first level, and the extra two sections cut for the audit became the second and third levels. All the sections of lymph nodes were examined by one of two examiners (SM and SP), at ×10 magnification. All suspicious areas were re‐examined at ×40 magnification. Positive cases were agreed by both examiners. Immunohistochemistry was performed for confirmation if necessary. The sizes of any additional metastases detected in levels two and three were recorded.
Results
A total of 101 cases of carcinoma of the upper gastrointestinal tract were examined: 30 oesophageal cancers (14 squamous carcinomas, 15 adenocarcinomas, 1 adenosquamous carcinoma), 34 oesophagogastric junction cancers (all adenocarcinomas) and 37 gastric cancers (all adenocarcinomas). The total number of lymph nodes harvested was 1279. The mean number of lymph nodes detected in all cases was 12.7 (range 1–38) (oesophagus mean = 13.1, range 2–28; gastro‐oesophageal junction mean = 14.3, range 1–35; gastric mean = 10.8, range 1–38). A total of 1143 lymph nodes were negative for tumour and were examined at an additional two levels. Table 1 gives a summary of the TNM stage, tumour differentiation and excision status.
Table 1 Summary of the TNM stage, tumour differentiation and excision status.
| Oesophagus (n = 30) | pT1 = 8 | pN0 = 20 | Well = 7 | R0 = 28 |
| pT2 = 8 | pN1 = 10 | Mod = 15 | R1+ = 2 | |
| pT3 = 13 | Poor = 8 | |||
| pT4 = 1 | ||||
| GOJ (n = 34) | pT1 = 2 | pN0 = 15 | Well = 3 | R0 = 31 |
| pT2 = 4 | pN1+ = 19* | Mod = 16 | R1+ = 3 | |
| pT3 = 28 | Poor = 15 | |||
| pT4 = 0 | ||||
| Stomach (n = 37) | pT1 = 9 | pN0 = 29 | Well = 5 | R0 = 36 |
| pT2 = 11 | pN1 = 4 | Mod = 14 | R1+ = 1 | |
| pT3 = 17 | pN2 = 4 | Poor = 18 | ||
| pT4 = 0 | pN3 = 0 |
GOJ, gastro‐oesophageal junction.
Well, Mod, Poor corresponds to the tumour differentiation; Mod, moderately differentiated.
R0, completed excised; R1+, incompletely excised.
*Classed as pN1+ as it was not always clear from the pathology report whether these were designated oesophageal or gastric for staging purposes.
Additional metastases were detected in levels 2 and 3 in 17 patients (table 2).
Table 2 Seventeen cases where metastatic deposits were detected on levels 2 and/or 3 that were not present on level 1.
| Case | Site | Type | Diff | pT | Total no. LN | No. +ve L1 | No. +ve L2/3 | Original pN → New pN |
|---|---|---|---|---|---|---|---|---|
| 1 | O | S | P | 3 | 13 | 7 | 8 | pN1 → pN1 |
| 2 | O | S | P | 3 | 3 | 0 | 1 | pN0 → pN1 |
| 3 | O | A | M | 1 | 20 | 2 | 3 | pN1 → pN1 |
| 4 | GOJ | A | M | 3 | 21 | 2 | 3 | pN1 → pN1 |
| 5 | GOJ | A | W | 3 | 7 | 0 | 1(ITC)* | pN0 → pN1/pN0(i+) |
| 6 | GOJ | A | P | 2 | 17 | 3 | 4 | pN1 → pN1 |
| 7 | GOJ | A | P | 3 | 16 | 2 | 3(ITC) | pN1 → pN1 |
| 8 | GOJ | A | P | 3 | 16 | 2 | 3 | pN1 → pN1 |
| 9 | GOJ | A | P | 2 | 22 | 5 | 10 | pN1 → pN1/2 |
| 10 | GOJ | A | M | 3 | 25 | 5 | 6 | pN1 → pN1 |
| 11 | GOJ | A | W | 3 | 5 | 0 | 1 | pN0 → pN1 |
| 12 | GOJ | A | P | 3 | 35 | 1 | 2 | pN1 → pN1 |
| 13 | G | A | P | 3 | 12 | 1 | 2 | pN1 → pN1 |
| 14 | G | A | M | 3 | 10 | 0 | 2(ITC)* | pN0 → pN1/pN0(i+) |
| 15 | G | A | P | 3 | 14 | 0 | 2 | pN0 → pN1 |
| 16 | G | A | W | 3 | 10 | 0 | 1 | pN0 → pN1 |
| 17 | G | A | P | 3 | 9 | 6 | 7 | pN1 → pN2 |
LN, lymph node; O, oesophagus; GOJ, gastro‐oesophageal junction; G, gastric; S, squamous cell carcinoma; A, adenocarcinoma; diff, differentiation; P, poorly; M, moderately; W, well; ITC, isolated tumour cells.
*Cases with isolated tumour cells classed as pN0 by the 1998 minimum dataset and as pN0(i+) in the sixth edition TNM classification.
In some patients more than one new metastatic deposit was detected. In total, levels 2 and 3 showed an additional 23 lymph node metastases (fig 1). In 6 patients this resulted in upstaging from N0 to N1, if using the present minimum datasets for oesophageal and gastric cancer. If the sixth edition of the TNM classification is used, 4 patients are upstaged from N0 to N1 and 2 from N0 to N0(i+). The remaining 11 patients were already stage N1. However one patient, with a gastric carcinoma, was upstaged from N1 to N2. Another patient, with a carcinoma of the oesophagogastric junction, would have also been upstaged from N1 to N2 if the tumour had been classed as gastric rather than oesophageal.
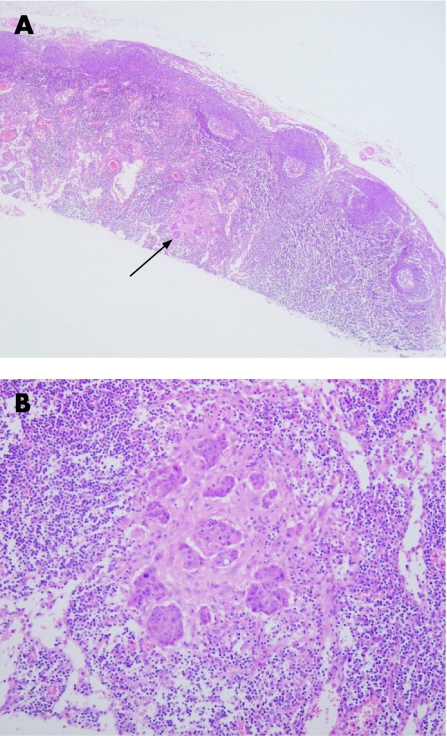
Figure 1 (A) Lymph node examined at level 2 illustrating a deposit of metastatic carcinoma not detected in level 1 (arrow). (B) Higher power magnification of metastatic deposit.
The size of the new lymph node deposits ranged from isolated tumour cells in 4 cases, metastases <0.2 mm in 6 cases, metastases 0.2–2 mm in 10 cases and metastases >2 mm in 3 cases. In the 4 cases of isolated tumour cells, immunohistochemistry (using a broad spectrum cytokeratin), was used for confirmation. In the sixth edition of the TNM classification, such cases are designated as pN0(i+).3
Discussion
Seventeen patients had additional lymph node metastases detected in levels 2 and 3. Eleven of the 17 patients were originally stage N1. In these patients the detection of extra metastases would probably not have altered management. However, the number of lymph nodes containing metastases has been related to prognosis in upper gastrointestinal tumours (the greater the number of involved lymph nodes the worse the prognosis).8,9,23 Six patients (6%) were originally stage N0 and were upstaged to N1/N0(i+) following examination of levels 2 and 3. This would have important significant implications for management and prognosis.
The present guidelines do not recommend the use of levels to detect metastatic tumour within lymph nodes from upper gastrointestinal cancers.8,9 In addition there is minimal guidance on how to sample lymph nodes. This results in different sampling methods being used by different pathologists. To ideally address this, the first question that needs answering is what size of metastatic deposit is significant in patients with upper gastrointestinal cancer. Only after this is answered can sensible guidelines be made on how to sample lymph nodes. Studies have examined the significance of total lymph node size, but there is no published evidence on the significance of size of metastatic deposit in gastric cancers.24 Studies on oesophageal cancers have shown that the size of metastatic deposit is prognostically significant.25,26 Patients with metastases measuring <4 mm2 showed no significant difference in survival compared to patients without lymph node metastases.25 At present, any deposit identified by H&E stain is regarded as involved. However the 6th edition of the TNM classification states that cases with no regional lymph node metastasis histologically, but with positive morphological findings for isolated tumour cells, should be designated as pN0(i+).3 In breast cancer, the size of the metastatic deposit is required in the histological report if only one lymph node is involved.15 Here metastases correspond to deposits >2 mm, micrometastases to deposits ⩽2 mm but >0.2 mm, and isolated tumour cells to deposits ⩽0.2 mm. Studies relating to prognosis and the presence of micrometastases in upper gastrointestinal tumours, diagnosed by immunohistochemistry, have been performed.17,18,19,20,21,22 Some of these studies show micrometastases to be significant, particularly in cases of gastric adenocarcinoma.17,18,22 In keeping with the results from this study, one paper reported upstaging from N0 to N1 in 7/75 patients with oesophageal cancer, stating that the deposits were of such size that they would have been picked up by H&E staining of one additional level.22 It is only with further studies regarding size of metastatic deposits that the prognostic implications of variably sized lymph node tumour deposits will be revealed.
The cases that were restaged from N0 to N1/N0(i+) were generally from cases with a low lymph node yield (⩽14 lymph nodes were originally harvested for each of these patients). The precise reasons for this finding are not known.
Possible recommendations following this audit include carrying out levels on all negative lymph nodes, on all negative lymph nodes in pN0 tumours or on all cases with a low lymph node yield (less than the recommended 15 lymph nodes required for accurate N staging of gastric tumours).10 Before recommending alterations in guidelines, the time and cost implications need to be considered, notwithstanding the uncertainties regarding the prognostic significance of metastases of different sizes. However, we believe that the small increase in workload required for triple levelling is justified by the increased accuracy in staging, at the very least in selected cases (pN0 tumours of low lymph node yield).
Take‐home messages
Examination of lymph nodes in upper gastrointestinal cancers at three levels detects more metastases than examination at one level.
Six of 101 patients were restaged from N0 to N1, based on present minimum datasets, with probable significant prognostic and management consequences.
Examination of three levels is justified, at the very least in pN0 tumours with a low lymph node yield (less than 15).
Standardisation of lymph node sampling is required. However, this can only be achieved with greater understanding of the significance of size of lymph node metastasis.
Acknowledgements
The authors would like to thank all the technical laboratory staff at South Manchester University Hospital for help in collecting the archival paraffin blocks and cutting of the levels.
Footnotes
Competing interests: None.
References
- 1. www.cancerresearchuk.org (accessed September 2006)
- 2.Blot W J, Devesa S S, Kneller R W.et al Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 19912651287–1289. [PubMed] [Google Scholar]
- 3.Sbin L, Wittekind C. eds. TNM classification of malignant tumours. 6th edn. New York: Wiley‐Liss 200260–68.
- 4.Eloubeidi M A, Desmond R, Arguedas M R.et al Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002951434–1443. [DOI] [PubMed] [Google Scholar]
- 5.Siewert J R, Stein H J, Feith M.et al Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001234360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendal W S. Lymph node‐based prognostics: limitations with individualized cancer treatment. Am J Clin Oncol 200629298–304. [DOI] [PubMed] [Google Scholar]
- 7.Siewert J R, Feith M, Werner M.et al Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000232353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon A.Standards and minimum data sets for reporting common cancers: gastric cancer. London: The Royal College of Pathologists, April, 2000
- 9.Mapstone N P.Standards and minimum data sets for reporting common cancers: oesophageal cancer. London: The Royal College of Pathologists, November, 1998
- 10.Novelli M R.Dataset for the histopathological reporting of gastric carcinoma, 2nd edn, draft. London: The Royal College of Pathologists, June 2006
- 11.Mapstone N P.Dataset for the histopathological reporting of oesophageal carcinoma, 2nd edn, draft. London: The Royal College of Pathologists, May 2006
- 12.Ibrahim N B N. Guidelines for handling oesophageal biopsies and resection specimens and their reporting. J Clin Pathol 20005389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R G, Compton C C. Protocol for the examination of specimens removed from patients with oesophageal cancer. A basis for checklist. The Cancer Committee, College of American Pathologists and the Task Force on the Examination of Specimens from Patients with Esophageal Cancer. Arch Pathol Lab Med 1997121925–929. [PubMed] [Google Scholar]
- 14.NHSBSP Pathology reporting in breast cancer screening. Publication no 3. Sheffield: NHSBSP Publications, 1995 (revised 1997),
- 15.NHSBSP Pathology reporting of breast disease. Publication no 58. Sheffield: NHSBSP Publications, January, 2005
- 16.Verrill C, Carr N J, Wilkinson‐Smith E.et al Histopathological assessment of lymph nodes in colorectal carcinoma: does triple levelling detect significantly more metastases? J Clin Pathol 2004571165–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda K, Adachi Y, Shiraishi N.et al Prognostic effect of lymph node micrometastasis in patients with histologically node‐negative gastric cancer. Ann Surg Oncol 20029771–774. [DOI] [PubMed] [Google Scholar]
- 18.Bonavina L, Ferrero S, Midolo V.et al Lymph node micrometastases in patients with adenocarcinoma of the esophagogastric junction. J Gastrointest Surg 19993468–476. [DOI] [PubMed] [Google Scholar]
- 19.Glickman J N, Torres C, Wang H H.et al The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer 1999861883–1885. [PubMed] [Google Scholar]
- 20.Sato F, Shimada Y, Li Z.et al Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg 200188426–432. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Ide H, Eguchi R.et al Clinical implications of lymph node micrometastasis in patients with histologically node‐negative (pN0) esophageal carcinoma. J Surg Oncol 200279224–229. [DOI] [PubMed] [Google Scholar]
- 22.Mueller J D, Stein H J, Oyang T.et al Frequency and clinical impact of lymph node micrometastasis and tumour cell microinvolvement in patients with adenocarcinoma of the esophagogastric junction. Cancer 2000891874–1882. [DOI] [PubMed] [Google Scholar]
- 23.Tachibana M, Yoshimura H, Kinugasa S.et al Clinicopathological factors correlated with number of metastatic lymph nodes in oesophageal cancer. Digest Liver Dis 200133534–538. [DOI] [PubMed] [Google Scholar]
- 24.Dhar D K, Kubota N, Kinukawa N.et al Prognostic significance of metastatic lymph node size in patients with gastric cancer. Br J Surg 2003901522–1530. [DOI] [PubMed] [Google Scholar]
- 25.Komori T, Doki Y, Kabuto T.et al Prognostic significance of the size of cancer nests in metastatic lymph nodes in human esophageal cancers. J Surg Oncol 20038219–27. [DOI] [PubMed] [Google Scholar]
- 26.Doi N, Imada T, Aoyama N.et al Prognostic significance of the carcinoma area in the thickest part of the lymph node. Hepatogastroenterology 200047728–732. [PubMed] [Google Scholar]