Abstract
The cyanobacteria Synechococcus sp. strain PCC 7942 and Synechococcus sp. strain PCC 6301 are very closely related and both have been designated by the binomial Anacystis nidulans. The only established difference between the two strains is the superior transformation properties of strain PCC 7942. Significant homology between the rRNA genes of these strains was demonstrated by the ability of an rRNA operon from strain PCC 6301, interrupted by a spectinomycin and streptomycin resistance marker, to transform strain PCC 7942 by recombining with and replacing an endogenous rRNA operon. Restriction fragment length polymorphism data indicated that the chromosomes of the two strains were conserved around the three psbA loci, the two rRNA operons, and the psbDI locus. However, multiple polymorphisms were detected downstream of the psbDII locus, identifying a DNA rearrangement such as an inversion, insertion, or deletion within the chromosome. Analysis of genome structure by pulsed-field gel electrophoresis of large NotI restriction fragments showed only two bands that were visibly shifted between the chromosomes of the two strains. These data support their very close genetic relationship and the feasibility of studying genes derived from strain PCC 6301 in the highly transformable PCC 7942 strain.
Full text
PDF

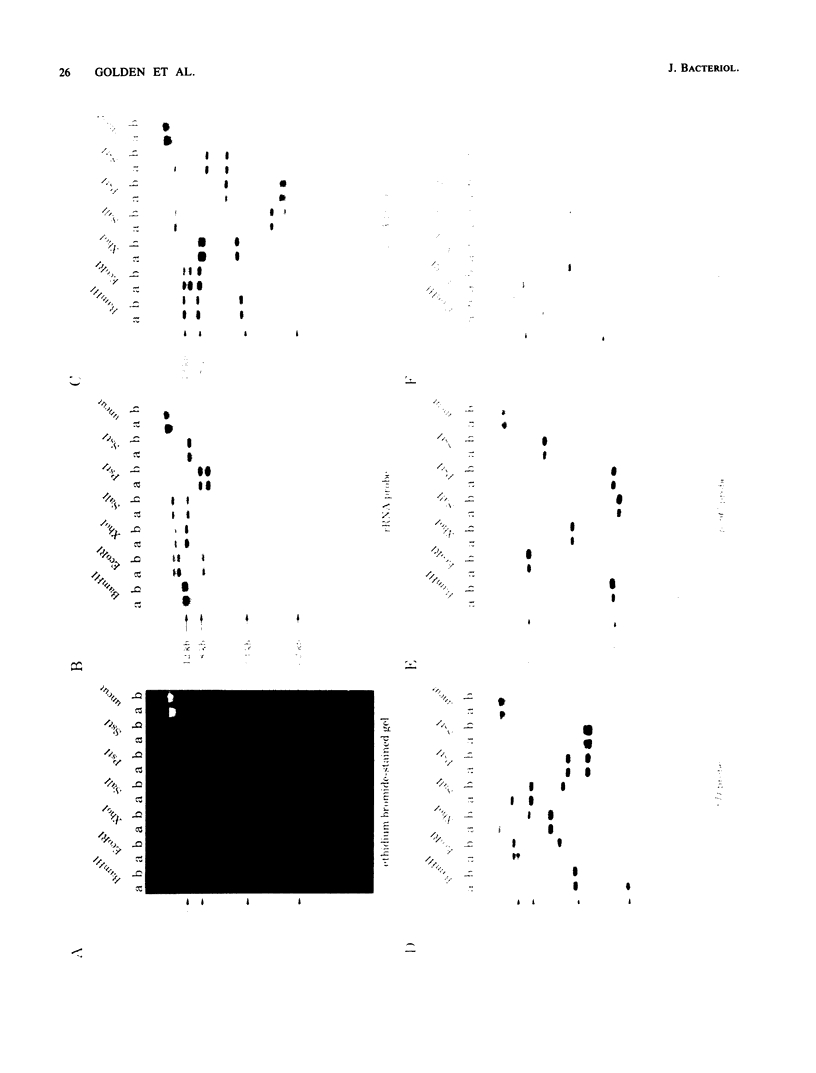



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. E., Lagarias J. C., Nagy J. O., Schoenleber R. W., Rapoport H., Klotz A. V., Glazer A. N. Phycobiliprotein-bilin linkage diversity. I. Structural studies on A- and D-ring-linked phycocyanobilins. J Biol Chem. 1986 May 25;261(15):6790–6796. [PubMed] [Google Scholar]
- Boussiba S., Dilling W., Gibson J. Methylammonium transport in Anacystis nidulans R-2. J Bacteriol. 1984 Oct;160(1):204–210. doi: 10.1128/jb.160.1.204-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1983 Sep;155(3):966–972. doi: 10.1128/jb.155.3.966-972.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Kalla S. R., Lind L. K., Lidholm J., Gustafsson P. Transcriptional organization of the phycocyanin subunit gene clusters of the cyanobacterium Anacystis nidulans UTEX 625. J Bacteriol. 1988 Jul;170(7):2961–2970. doi: 10.1128/jb.170.7.2961-2970.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C. J., Teeuwsen V. J., Janssen M. J., van Arkel G. A. Cloning of a third nitrate reductase gene from the cyanobacterium Anacystis nidulans R2 using a shuttle cosmid library. Gene. 1984 Nov;31(1-3):109–116. doi: 10.1016/0378-1119(84)90200-2. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C. J., van Arkel G. A. Host-vector systems for gene cloning in cyanobacteria. Methods Enzymol. 1987;153:199–215. doi: 10.1016/0076-6879(87)53054-3. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Reddy K. J., Sherman L. A. Nucleotide sequence of the gene from the cyanobacterium Anacystis nidulans R2 encoding the Mn-stabilizing protein involved in photosystem II water oxidation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8230–8234. doi: 10.1073/pnas.84.23.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A., Hinterstoisser B., Riedler M., Muchl R., Nitschmann W. H. Exogenous energy supply to the plasma membrane of dark anaerobic cyanobacterium Anacystis nidulans: thermodynamic and kinetic characterization of the ATP synthesis effected by an artificial proton motive force. Arch Biochem Biophys. 1986 May 15;247(1):40–48. doi: 10.1016/0003-9861(86)90530-8. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Phycobilisome-associated glycoproteins in the cyanobacterium Anacystis nidulans R 2. FEBS Lett. 1987 May 11;215(2):209–214. doi: 10.1016/0014-5793(87)80147-3. [DOI] [PubMed] [Google Scholar]
- Samuelsson G., Lönneborg A., Gustafsson P., Oquist G. The Susceptibility of Photosynthesis to Photoinhibition and the Capacity of Recovery in High and Low Light Grown Cyanobacteria, Anacystis nidulans. Plant Physiol. 1987 Feb;83(2):438–441. doi: 10.1104/pp.83.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surányi G., Korcz A., Pálfi Z., Borbély G. Effects of light deprivation on RNA synthesis, accumulation of guanosine 3'(2')-diphosphate 5'-diphosphate, and protein synthesis in heat-shocked Synechococcus sp. strain PCC 6301, a cyanobacterium. J Bacteriol. 1987 Feb;169(2):632–639. doi: 10.1128/jb.169.2.632-639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel T., Wolk C. P. Conjugal transfer of plasmids to cyanobacteria. Methods Enzymol. 1987;153:232–243. doi: 10.1016/0076-6879(87)53056-7. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]