Abstract
Background
Endoscopic ultrasound guided fine needle aspiration biopsy (EUS‐FNA) has proven to be an effective diagnostic modality for the detection and staging of pancreatic malignancies. In recent years EUS‐FNA has also been used to diagnose lesions of non‐pancreatic sites such as structures in close proximity to the gut wall within the mediastinum, abdomen, pelvis and retro‐peritoneum.
Aims
To evaluate experience with EUS‐FNA of non‐pancreatic sites at a large university medical centre.
Methods
The study cohort included 234 patients who underwent EUS‐FNA of 246 lesions in non‐pancreatic sites (122 peri‐pancreatic and coeliac lymph nodes; 9 peri‐pancreatic masses; other sites: mediastinum 12, gastric 25, liver 27, oesophagus 17, duodenum/colon/rectum 15, retro‐peritoneum 8, lung 7, miscellaneous 4).
Results
The cytology diagnoses were classified as non‐neoplastic/reactive in 82 (33%), atypical/suspicious for malignancy in 25 (10%), malignant in 86 (35%) and non‐diagnostic in 53 (22%) cases. Surgical pathology follow‐up was available in 75 (31%) cases. Excluding the non‐diagnostic cases there were 7 false negative and 3 false positive cases. The sensitivity, specificity and positive predictive value of EUS‐FNA in the diagnosis of lesions of non‐pancreatic sites was 92%, 98% and 97%, respectively.
Conclusions
EUS‐FNA can be effectively used as a diagnostic modality in the diagnosis of lesions from non‐pancreatic sites.
Keywords: endoscopy, ultrasound, fine needle biopsy
Endoscopic ultrasonography (EUS) has proven itself as a superior tool in visualising, identifying and characterising the extent of lesions of the gastrointestinal (GI) tract and adjacent structures.1,2,3 These include the liver, pancreas, and structures within the mediastinum, abdomen and pelvis. It is also used as a diagnostic tool for the evaluation of submucosal masses of the upper GI tract and the rectosigmoid, for locating pancreatic endocrine tumours, and for the assessment of vascular disease.1,2,3,4,5,6,7,8,9
EUS, even though it accurately stages GI malignancies, cannot alone reliably differentiate benign from malignant lesions or neoplastic from inflammatory processes.8,9 Consequently, pathological examination is often required to establish a definitive diagnosis for further clinical management.10,11,12,13 In recent years, advances in technology have permitted the performance of fine needle aspiration (FNA) biopsy under EUS guidance.7 A curvilinear echoendoscope, operating at 5–7.5 MHz permits continuous, real time imaging and guidance for the sampling of lesions using 19 or 25 gauge needles. The ability to obtain cytological material under direct visualisation adds a new dimension to the diagnostic usefulness of this technique as it offers an opportunity for prompt and accurate diagnosis.6,14 Thus, EUS‐guided FNA (EUS‐FNA) has become a standard procedure in many institutions in diagnosing pancreatic, GI and mediastinal malignancies.6,15,16
The role of EUS‐FNA in the diagnosis of pancreatic malignancies is well established. However, there have been only a few reports investigating the success of EUS directed FNA in non‐pancreatic sites. These include intramural and extramural structures of the GI tract such as those surrounding the gut wall within the mediastinum, abdomen, pelvis and retroperitoneum.17,18,19,20,21,22,23,24,25,26,27,28,29,30
In this study, we report our experience with the role of EUS‐FNA in the diagnosis of lesions of non‐pancreatic sites at a large university medical centre.
Materials and methods
Patients
A retrospective chart review was performed, which included a computerised search of patients undergoing EUS‐FNA at the Hospital of University of Pennsylvania from January 1999 to September 2004. The 485 consecutive patients undergoing EUS‐FNA and brushing cytology of both pancreatic and non‐pancreatic sites were prospectively included in this study (table 1). The patients had been referred for EUS guided biopsy based on the need to evaluate suspicious GI, pelvic, pancreatic, hepatobiliary, or mediastinal lesions or for the staging of known GI or pulmonary malignancies.
Table 1 Characteristics of patients and organs/sites sampled by endoscopic ultrasound guided fine needle aspiration.
| Site | Cases (n) | Patients (n) | Sex (M/F) | Mean age (y) | Size (cm) | Mean passes |
|---|---|---|---|---|---|---|
| Lymph nodes | 122 | 116 | 75/41 | 63.6 | 1.8 | 2.5 |
| Peri‐pancreatic | 9 | 9 | 7/2 | 65.9 | 3.2 | 2.5 |
| Hepatobiliary | 27 | 25 | 13/12 | 65.6 | 3.7 | 2.1 |
| Oesophagus | 17 | 17 | 11/6 | 69.4 | 5.4 | 2.4 |
| Stomach | 25 | 22 | 13/9 | 65.0 | 5.2 | 2.4 |
| Mediastinum | 12 | 11 | 7/4 | 62.0 | 3.3 | 2.7 |
| Retroperitoneum | 8 | 8 | 6/2 | 58.1 | 5.0 | 3.8 |
| Lung | 7 | 7 | 4/3 | 73.5 | 4.2 | 2.4 |
| Gastrointestinal tract | 15 | 15 | 6/9 | 58.5 | 2.5 | 2.6 |
| Miscellaneous* | 4 | 4 | 2/2 | 71.3 | 10.0 | 1.8 |
*Including intra‐abdominal mass and umbilical nodule.
Informed consent was obtained from all patients. The institutional review board approved the performance of the biopsy procedure and data ascertainment. One of the three attending gastroenterologists performed all EUS‐FNA procedures. Pancreatic lesions and brushing cytology were excluded from the study. A total of 234 patients (144 men and 90 women) with a mean age of 64.3 years (range 13–95 years) underwent EUS‐FNA of 246 lesions in non‐pancreatic sites using a curved linear array echoendoscope.
Methods
In all patients EUS was planned to assist in staging a suspected neoplasm, to further evaluate a mass lesion, to detect tumour recurrence, for routine tumour surveillance or to obtain a specific cytological diagnosis.
Eighty‐seven patients had previous or concurrent non‐diagnostic or equivocal procedures (pancreatic aspiration, n = 24; brushing cytology, n = 17; fluid cytology, n = 7; bronchial washing, n = 3; conventional forceps biopsies, n = 32; and percutaneous liver biopsy, n = 4) and 50 patients had previous or concurrent procedures that were diagnosed as either malignant or suspicious (pancreatic aspiration, n = 21; brushing cytology, n = 8; fluid cytology, n = 4; bronchial washing, n = 1; and conventional forceps biopsies, n = 16).
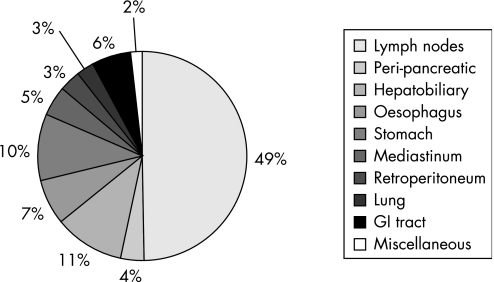
FNA sites included lymph nodes (n = 122), peri‐pancreatic lesions (n = 9), hepatobiliary (n = 27), oesophagus (n = 17), gastric (n = 25), mediastinum (n = 12), retroperitoneum (n = 8), lung (n = 7), GI tract (n = 15) and miscellaneous including intra‐abdominal masses and umbilical nodule (n = 4) (table 1, fig 1).
Figure 1 Distribution of endoscopic ultrasound guided fine needle aspiration by sampled site/organ.
The size of the lesions ranged from 0.5 cm to 18 cm (mean 2.95 cm) in 114 cases. In 31 cases, no discrete mass was discernible. The size was not available in the remaining 101 cases. On average, 2.5 needle passes (range 1–6 passes) were made per lesion.
Indications for EUS guided biopsy
The indications for EUS guided biopsy included a pancreatic mass seen on either CT, endoscopic retrograde cholangio‐pancreaticography (ERCP) or EUS; mediastinal mass or lymphadenopathy detected by CT scan; hepatobiliary mass seen by CT, ERCP or EUS; and submucosal or extrinsic GI mass seen on endoscopy or CT.
Technique
Endosonography
EUS‐FNA was performed in the endoscopy suite under conscious sedation.
Those patients who were suspected to have or had previously been found radiologically or endoscopically to have luminal abnormalities underwent standard, forward‐viewing endoscopy to evaluate mucosal lesions before undergoing EUS. Evaluation of the target lesion and/or staging of tumours were initially performed with a radial scanning echoendoscope (GF‐UM160, Olympus America, Melville, NY, USA).
EUS‐FNA was then performed using the curved linear array echoendoscope (GF‐UM160, Olympus America).
This system can also perform pulse and colour Doppler studies, which can enhance the identification of structures and aid in avoiding vessels during FNA. As the scanning plane is in the long axis of the instrument, it allows for real time visualisation of the biopsy needle. A biopsy channel permits the passage of the FNA needle into the scanning plane of the instrument.
At the time of EUS, attention was directed to determine the staging of the lesion (TNM), the echo‐characteristics of each lesion, the orientation relative to the adjacent structures, and the distance between the lesion and overlying mucosa during endosonography.
Aspiration
After completion of the diagnostic and staging components of the endosonographic examination and identification of a lesion of interest, FNA specimens were obtained. A cytopathologist was present on‐site for all cases to process the material, assess the adequacy of the specimen, and to render a preliminary on‐site diagnostic impression. In all cases a 22‐gauge needle with stylet was utilised (HAJ‐30, Olympus America; or EUSN‐3, Cook Medical, Bloomington, IN, USA). The needle was advanced into the lesion under real time endosonographic visualisation and inserted into the mass, with a maximum depth of penetration of 50 mm. The needle stylet was then removed from the assembly. When the needle tip was seen to lie within the lesion, continuous suction was applied with a 20 ml plastic syringe. At the same time, the needle was moved back and forth within the lesion with 3–5 mm movements, while observing on the ultrasound console screen. Suction was then released, and the needle was withdrawn.
The contents of the needle were expressed onto a glass slide and direct smears were prepared in the endoscopy suite with an average of two slides made per pass. Half of the slides were air‐dried and processed with the Diff‐Quik (Harleco, Gibbstown, NJ, USA) stain and immediately examined. The remaining half were immediately placed in 95% alcohol for subsequent Papanicolaou staining. The Diff‐Quik stained slides were assessed for specimen adequacy for each pass. Passes were made until an adequate specimen was obtained, with a maximum of 6 passes (average 2.5 passes).
The needle was flushed with Normosol (Abbott Laboratories, North Chicago, IL, USA), and the stylet was passed through the needle to recover remaining material; this was collected in Normosol for subsequent preparation of cellulose membrane filter (Millipore Corporation, Bedford, MA, USA) and a cellblock, when possible. If an undifferentiated tumour (mesenchymal, lymphoreticular) was within the differential diagnosis, additional studies were performed to arrive at a diagnosis. These included immunophenotyping by flow cytometry and microbiology for culture. Immunoperoxidase staining was performed on cell block material, direct smears, or cytospin preparations using standard immunoperoxidase technique.
A specimen was considered adequate/satisfactory by the cytopathologist if there were a sufficient number of representative cells present from the target lesion. Cytopathology diagnoses were categorised as follows: malignant/neoplastic, atypical/suspicious, benign/reactive, and non‐diagnostic (ND). The non‐diagnostic aspirates contained a qualifier regarding the reason the FNA specimen was considered non‐diagnostic (eg, scant cellularity, not representative of the lesion, acellular, cellular degeneration).
Cytohistological correlation
The histopathological follow‐up was available in 77 cases (31.3%). In the remaining 169 cases, clinical follow‐up of 1–69 months was reviewed. However, this data was not used to calculate the sensitivity and specificity in the present study.
Results
Of the 246 FNA specimens obtained from non‐pancreatic sites by EUS, 193 were considered diagnostic (78%). The cytological diagnoses included malignant/neoplasm in 86 cases (35%), non‐neoplastic or reactive in 82 (33%), atypical/suspicious for malignancy in 25 cases (10%), and 53 were considered non‐diagnostic (22%).
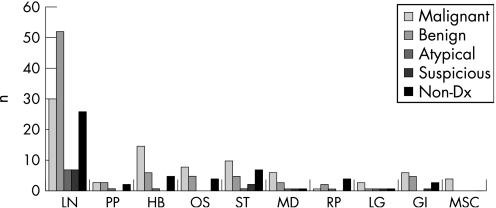
The final cytological diagnoses correlated with the on‐site interpretation/diagnosis in 154 cases (63%) and did not correlate in the remaining 92 cases (37%). This was explained by paucity of diagnostic material in the on‐site prepared Diff Quick slides with subsequent recovery of diagnostic material from the Normosol or Papanicolaou stained slides, or was due to further characterisation of the aspirate by flow‐cytometry and special staining of the cell block or cytospin preparations. Excluding the non‐diagnostic cases, there were 7 false negative and 3 false positive cases. The calculated sensitivity, specificity and positive predictive value of EUS‐FNA in the diagnosis of lesions of non‐pancreatic sites was 92%, 98% and 97% respectively. Table 2 and fig 2 summarise the results.
Table 2 Cytological diagnosis, histological follow‐up and operating characteristics of endoscopic ultrasound guided fine needle aspiration (EUS‐FNA) by sampled sites.
| Site | EUS‐FNA diagnosis M/B/A/S/ND (n) | SP F/U (n) | FP or FN (n) | Sensitivity/specificity (%) |
|---|---|---|---|---|
| Lymph nodes | 30/52/7/7/26 | 19 | FP = 1 | 100/99 |
| Peri‐pancreatic | 3/3/1/0/2 | 5 | FN = 2 | 60/100 |
| Hepatobiliary | 15/6/1/0/5 | 7 | FN = 1 | 94/100 |
| Oesophagus | 8/5/0/0/4 | 7 | FN = 1 | 89/100 |
| Stomach | 10/5/1/2/7 | 19 | FN = 1 | 91/100 |
| Mediastinum | 6/3/1/1/1 | 2 | 0 | 100/100 |
| Retroperitoneum | 1/2/1/0/4 | 3 | FN = 1 | 50/100 |
| Lung | 3/1/1/1/1 | 2 | 0 | 100/100 |
| GI tract | 6/5/0/1/3 | 10 | FP = 1 | 100/86 |
| Miscellaneous | 4/0/0/0/0 | 1 | 0 | 100/100 |
M, malignant; B, benign; A, atypical; S, suspicious; ND, non‐diagnostic; SP F/U, surgical pathology follow‐up; FP, false positive; FN, false negative.
Figure 2 Distribution of endoscopic ultrasound guided fine needle aspiration diagnoses by sampled site/organ. LN, lymph nodes; PP, peri‐pancreatic; HB, hepatobiliary; OS, oesophagus; ST, stomach; MD, mediastinum; RP, retroperitoneum; LG, lung; GI, GI tract; MSC, miscellaneous; n, total number of cases; Non‐Dx, non‐diagnostic.
Lymph nodes
A total of 122 lymph nodes underwent EUS‐FNA in 116 patients (41 women and 75 men, mean age 64 years). The average size of the lymph nodes was 1.83 cm (range 0.5–4 cm).
The lymph nodes were coeliac (n = 23), peri‐duodenal (n = 2), rectal (n = 2), peri/para‐oesophageal (n = 9), peri‐gastric/gastric/gastro‐splenic (n = 14), portal (n = 1), subcarinal (n = 4), para‐tracheal (n = 2), aortopulmonary window (n = 2), mediastinal (n = 21), peri‐pancreatic (n = 38), retroperitoneal (n = 3) and abdominal (n = 1).
Thirty‐three patients had prior diagnosis of malignancy/neoplasm (oesophageal cancer, n = 10; gastric adenocarcinoma, n = 1; breast cancer, n = 2; pancreatic cancer, n = 2; oropharyngeal cancer, n = 1; melanoma, n = 1; renal cell cancer, n = 1; hepatocellular cancer, n = 2; non‐Hodgkin lymphoma, n = 5; colorectal cancer, n = 3; Hodgkin lymphoma, n = 1; small cell lung carcinoma, n = 2; and non‐small cell lung carcinoma, n = 2).
A diagnosis of malignancy was established by EUS‐FNA in 30 patients (24%), 52 cases (43%) were diagnosed as non‐neoplastic/benign, 7 cases (6%) as atypical, 7 cases (6%) as suspicious for malignancy and 26 cases (21%) as non‐diagnostic. The histopathological follow‐up was available in 8 of the 30 neoplastic/malignant cases (27%). There was one false positive case: FNA of a peri‐pancreatic lymph node diagnosed as tumour present with neuroendocrine features. However, a subsequent distal pancreatectomy revealed chronic pancreatitis with pseudocyst formation, and no tumour was detected in 19 peri‐pancreatic lymph nodes examined.
Nine of the 52 non‐neoplastic FNAs had histological follow‐up (17%). There was no false negative aspirate. One of the 14 atypical/suspicious FNA had subsequent biopsy proven adenocarcinoma followed by a Whipple procedure showing the same. In three cases of coeliac lymph nodes diagnosed as atypical/suspicious, EUS‐FNA of the pancreas was done at the same time, showing adenocarcinoma. One atypical FNA of peri‐rectal lymph node had a simultaneous aspiration of the rectal lesion, which showed involvement by Hodgkin lymphoma.
Two of the 26 non‐diagnostic aspirates on cytology had adequate histological follow‐up (8%). The first was an 8 mm coeliac lymph node aspirate in a 61‐year‐old woman who presented with jaundice and a pancreatic head mass. The pancreatic mass aspirate showed a benign cytology. The coeliac lymph node aspirate, though satisfactory, showed only benign glandular cells; no lymphoid tissue was present and the FNA was considered non‐diagnostic. A subsequent Whipple procedure in the patient showed a moderately differentiated mucinous adenocarcinoma of the head of pancreas with extensive perineural/lymphovascular invasion and involvement of 2/5 peri‐pancreatic lymph nodes, 1 common bile duct lymph node and 1 mesenteric lymph node.
The second case was of a 2 cm perigastric lymph node aspirate in a 60‐year‐old man with a history of Hodgkin lymphoma (nodular sclerosis type) who later presented with a perigastric lymph node, speculated lung mass and a mediastinal lymph node. EUS‐FNA of the perigastric lymph node showed benign glandular epithelium and a few lymphocytes, and was read as unsatisfactory (not representative of the lesion). A subsequent exploratory laparotomy with excisional biopsy from the perigastric lymph node showed recurrent classical Hodgkin lymphoma, nodular sclerosis type.
The calculated sensitivity and specificity of EUS‐FNA of malignant lymph nodes was 100% and 99%, respectively. The positive predictive value was 97%.
Peri‐pancreatic lesions
Nine peri‐pancreatic lesions in 9 patients (7 men and 2 women) with a mean age of 66 years (range 56–80 years) were aspirated. The mean size of the lesions was 3.23 cm (range 2.2–5 cm). Three cases were diagnosed as malignant/neoplasm (33%), 1 as atypical/suspicious (11%), 3 as benign (33%) and 2 as non‐diagnostic (23%).
Malignant/neoplasm diagnoses included neuroendocrine neoplasm (n = 2) and GI stromal tumour (GIST) (n = 1). The latter diagnosis was confirmed by immunostains for CD34 and C‐KIT.
Two neuroendocrine neoplasm cases had histopathological follow‐up, including a distal pancreatectomy and an enucleation procedure, both of which showed peri‐pancreatic neuroendocrine tumours.
The case of GIST had no histological follow‐up. There were no false positive diagnoses.
All three benign peri‐pancreatic aspirates had histopathological follow‐up, which included two Whipple procedures and one excisional biopsy. There were two false negative cases. Whipple procedures revealed poorly differentiated adenocarcinoma invading into peri‐pancreatic tissue. The excision biopsy of the peri‐pancreatic mass (at the coeliac/supra‐mesenteric axis) revealed fibrous tissue with mixed inflammatory infiltrate, reactive lymphoid tissue and no tumour.
The one atypical/suspicious peri‐pancreatic aspirate showed rare atypical cells suspicious but not diagnostic for malignancy. This patient had a history of transitional cell carcinoma, which was not available for review. Scant cellularity of the tissue limited further characterisation on cytology.
The calculated sensitivity and specificity of EUS‐FNA of malignant peri‐pancreatic lesions was 60% and 100%, respectively. The positive predictive value was 100%.
Hepatobiliary
Twenty‐seven hepatobiliary lesions underwent EUS‐FNA in 25 patients (13 men and 12 women). The mean age for these patients was 66 years (range 36–91 years). The average size of the lesions sampled was 3.7 cm (range 0.9–10 cm).
These included liver masses (n = 23), porta hepatis mass (n = 1) and biliary aspirations (n = 3). Eight of the liver masses were located in the left lobe, 2 in the right lobe, 1 in the hilar area and 12 as multiple liver lesions. The biliary aspirations included 2 bile duct strictures and 1 bile duct mass.
Eight patients had a known history of malignant disease prior to the EUS‐FNA. This included: oesophageal adenocarcinoma (n = 1), metastatic pancreatic cancer (n = 1), breast cancer (n = 3), pancreatic adenocarcinoma (n = 1), hepatocellular cancer (n = 1), cholangiocarcinoma (n = 1), renal cell cancer (n = 1), colon cancer (n = 1) and mucinous adenocarcinoma of the appendix (n = 1).
Two of these patients had primary carcinoma of more than one organ (proven prior to the EUS‐FNA of their current hepatobiliary lesion). One patient had a remote history of colon cancer with a recent history of breast and renal cancer and the other patient had a history of breast cancer and a mucinous adenocarcinoma of the appendix with colon metastases.
The FNA diagnoses were malignant in 15 (56%), benign in 6 (22%), atypical in 1 (4%) and non‐diagnostic in 5 (18%) cases.
Neoplastic lesions included: metastatic poorly differentiated carcinoma (n = 8), metastatic adenocarcinoma (n = 3), metastatic squamous cell carcinoma (n = 1), hepatocellular carcinoma (n = 1), neuroendocrine neoplasm (n = 1) and poorly differentiated neuroendocrine carcinoma, non‐small cell type (n = 1).
Two of 15 neoplastic/malignant aspirates had subsequent biopsy proven malignancy (13%): a Whipple procedure with a liver biopsy showing metastatic islet cell carcinoma consistent with a pancreatic primary; and a CT‐guided liver biopsy showing metastatic poorly differentiated carcinoma compatible with a breast primary.
The remaining 13 malignant/neoplastic cases had no histopathological follow‐up (87%). This group included a patient with a diagnosis of hepatocellular carcinoma on FNA and a subsequent abdominal CT abdomen examination showing unresectable multifocal primary tumour of liver involving all the liver segments with peritoneal implants and mesenteric adenopathy. Six patients with simultaneous aspiration of the pancreas and liver showed adenocarcinoma (n = 4) and poorly differentiated neuroendocrine carcinoma (n = 2). One patient with history of breast cancer and mucinous adenocarcinoma of the appendix with metastasis to the colon showed squamous cell carcinoma of the liver on FNA. This patient also had a simultaneous bile duct brushing diagnosed as squamous cell carcinoma. There were no false positive diagnoses.
Three of the six benign aspirates had histopathological follow‐up and EUS‐FNA of biliary lesions. The surgical procedures included bile duct biopsy in two cases and hepatic resection in one case. There was one false negative case: a 58‐year‐old woman who presented with history of primary sclerosing cholangitis showed no evidence of malignancy on EUS‐FNA. A subsequent biliary stricture brushing was diagnosed as adenocarcinoma. Exploratory laparotomy with liver biopsies was negative; however, hepatic resection showed the presence of cholangiocarcinoma, sclerosing variant, arising in an extrahepatic bile duct.
The one atypical/suspicious aspirate was from a porta hepatis mass. The cytology showed reactive hepatocytes with focal atypia suspicious for tumour. This was the case of a 56‐year‐old man with history of hepatitis C and hepatocellular carcinoma and status post‐liver transplant. The patient presented with raised liver function tests, a 4 cm mass in the porta hepatis and enlarged peri‐pancreatic lymph nodes. The EUS‐FNA of the peri‐pancreatic lymph node revealed malignant epithelial neoplasm favouring recurrent hepatocellular carcinoma. A subsequent core biopsy of the porta hepatis mass confirmed the diagnosis of hepatocellular carcinoma.
One of the 5 non‐diagnostic aspirates on cytology had adequate histological follow‐up. This was the case of a 76‐year‐old woman who presented with an abdominal mass, a right renal mass and a 10 cm liver mass. The EUS‐FNA of the liver mass showed predominantly blood with rare squamous cells, macrophages and acellular material and was thus unsatisfactory due to scant cellularity. Subsequent excision of the abdominal mass showed a malignant GIST arising from the duodenum. The right kidney mass on histology was a papillary renal cell carcinoma, eosinophilic variant. The biopsy of the liver mass showed a vascular lesion, type undetermined (not certain if a hemangioma). This liver mass was both histologically and immunohistochemically distinct from either GIST or papillary renal cell carcinoma.
The calculated sensitivity and specificity of EUS‐FNA of malignant hepatobiliary lesions was 94% and 100%, respectively. The positive predictive value was 100%.
Oesophagus
Seventeen oesophageal lesions in 17 patients (11 men and 6 women) with a mean age of 69 years (range 51–87 years) were aspirated. The mean size of the lesions was 5.35 cm (range 2–5.7 cm).
Oesophageal lesions included: peri/para‐oesophageal masses (n = 6), oesophageal masses (n = 10), and oesophagus anastomotic site stricture (n = 1).
Malignant disease was confirmed prior to the EUS‐FNA in 7 patients. This included
oesophageal adenocarcinoma (n = 4), oesophageal squamous cell carcinoma (n = 1), adenocarcinoma of lung (n = 1) and breast carcinoma (n = 2).
The cytological diagnoses included: 8 malignant cases (47%), 5 benign/non‐neoplastic cases (29%) and 4 non‐diagnostic cases (24%).
Malignant lesions included: adenocarcinoma (n = 3), squamous cell carcinoma (n = 3) and poorly differentiated carcinoma (n = 2). Among these 8 neoplastic lesions, 2 were recurrent primary oesophageal adenocarcinomas, 1 was a recurrent primary oesophageal squamous cell carcinoma and 1 was a breast carcinoma metastasis to oesophagus. The remaining 4 lesions were primary oesophageal cancers. Histopathological follow‐up was available in 2 cases which showed recurrent adenocarcinoma and squamous cell carcinoma on biopsies.
In the remaining 6 malignant cases without histopathological follow‐up, clinical information was available in 4 cases. A patient with a history of adenocarcinoma of the oesophagus, presented a few years later with a peri‐oesophageal soft tissue mass, which on EUS‐FNA showed adenocarcinoma. Positron emission tomography scan showed coeliac axis nodal involvement consistent with metastases. Subsequent CT scans of the thorax also showed recurrent tumour evidenced by increasing subcarinal mediastinal mass and a right adrenal metastasis. A patient with a para‐oesophageal mass diagnosed as squamous cell carcinoma had a subsequent oesophagogram which showed oesophageal‐pulmonary bronchial fistula consistent with lung cancer invading the oesophagus. A patient with a history of breast carcinoma presented with an extrinsic oesophageal mass. The EUS‐FNA of this lesion showed adenocarcinoma morphologically compatible with a breast primary.
A patient presented with an oesophageal mass and an enlarged peri‐oesophageal lymph node, both aspirated during the same procedure. Squamous cell carcinoma was seen in both specimens on cytology. There were no false positive diagnoses.
Three of the five non‐neoplastic/benign aspirates had histopathological follow‐up. These lesions included aspirations from the gastro‐oesophageal junction, a submucosal oesophageal mass and a distal oesophageal brushing and aspiration.
There was one false negative case. A distal oesophageal aspiration in a 51‐year‐old man with oesophageal stricture showed reactive squamous epithelium on cytology, but a subsequent biopsy showed squamous mucosa with markedly atypical epithelium and chronic inflammation, suspicious for but not diagnostic of malignancy. A diagnosis of oesophageal adenocarcinoma was confirmed later.
Two of the four non‐diagnostic oesophageal aspirates on cytology had histological follow‐up. The first was a 5.7 cm lower oesophageal mass in a 59‐year‐old woman who presented with worsening abdominal pain and dysphagia. The EUS‐FNA of the lower oesophageal mass showed only squamous cells, mucin and macrophages and was thus unsatisfactory due to scant cellularity. A subsequent resection of the lower oesophageal intramural mass showed a 7 cm leiomyoma. Also the gastro‐oesophageal junction biopsy showed mild chronic inflammation with no intestinal metaplasia or dysplasia.
The second was from a 59‐year‐old man who presented with progressive dysphagia. A subsequent endoscopy revealed a large submucosal mass in the cervical region of the oesophagus. EUS‐FNA of this mass showed predominant squamous epithelium on cytology and was thus unsatisfactory due to scant cellularity. A subsequent excision of the submucosal oesophageal mass showed a 3 cm leiomyoma.
The calculated sensitivity and specificity of EUS‐FNA of malignant oesophageal lesions was 89% and 100%, respectively. The positive predictive value was 100%.
Gastric lesions
Twenty‐two patients (13 men and 9 women) underwent EUS‐FNA of 25 gastric lesions. The mean age of the patients was 65 years (range 27–88 years). The mean size of the lesions was 5 cm (range 1.5–18 cm).
The gastric lesions included: gastric mass (n = 17), retrogastric mass (n = 2), gastro‐oesophageal junction mass (n = 2), gastric ulcer (n = 2) and thickened gastric wall (n = 2). Malignant disease was confirmed prior to the procedure in 4 patients: breast carcinoma (n = 1), non‐Hodgkin lymphoma (n = 1), non‐small cell lung cancer (n = 1) and oesophageal adenocarcinoma (n = 1).
The cytology diagnoses were malignant in 10 (40%), benign/non‐neoplastic in 5 (20%), atypical/suspicious in 3 (12%) and non‐diagnostic in 7 (28%) cases.
The malignant/neoplastic diagnoses included: primary gastric adenocarcinoma (n = 1); metastatic adenocarcinoma (n = 1); poorly differentiated adenocarcinoma (n = 1); GIST (n = 3); non‐Hodgkin lymphoma, including mantle cell lymphoma, B‐cell marginal zone lymphoma and B‐cell large cell lymphoma (n = 3); and poorly differentiated neoplasm (n = 1). Seven of the 10 malignant/neoplastic cases had histopathological follow‐up. There were no false positive cases.
Four of the five benign aspirates had surgical evaluation of their corresponding tissues, which included three biopsies and one surgical resection (laparoscopic partial gastrectomy). All the three gastric mass biopsies showed chronic inflammation and focal intestinal metaplasia with no evidence of dysplasia or malignancy. This correlated with their corresponding benign cytological diagnoses.
There was one false negative diagnosis. In this case laparoscopic partial gastrectomy showed a 1.7 cm GIST.
Histopathological follow‐up was available in 2 of 3 cases diagnosed as atypical/suspicious on EUS‐FNA. There was one false negative case. In the first case the gastric mass biopsy and a subsequent gastrectomy established the presence of a poorly differentiated adenocarcinoma. In the second case, biopsy of the gastric lesion showed chronic inflammation, focal ulceration and fungal colonisation.
Six of the seven non‐diagnostic gastric aspirates on cytology had histological follow‐up available. One was an endoscopic aspirate of a submucosal gastric nodule in a 70‐year‐old woman. On cytology, this specimen showed few glandular cells and was considered unsatisfactory due to scant cellularity. A subsequent excisional biopsy was also non‐diagnostic and not representative of the lesion seen endoscopically. No repeat biopsy was available.
The second was the case of a 71‐year‐old man with history of diabetes who presented with progressive dysphagia. EUS‐FNA of a gastro‐oesophageal junction mass showed benign squamous and glandular cells on cytology and was considered unsatisfactory (not representative of the lesion). A subsequent gastro‐oesophageal junction, antral and gastric body biopsy showed mild chronic inflammation with focal intestinal metaplasia and no dysplasia.
The third was the case of a 56‐year‐old man with history of Waldenstrom's macroglobulinaemia, low grade B cell lymphoma, status post‐chemotherapy with Richter's transformation, and myelodysplastic syndrome who presented with upper GI bleeding. Endoscopic examination showed thickening of the gastric wall at the antral body junction on the greater curvature. EUS‐FNA of this lesion showed few groups of benign appearing glandular cells and was considered unsatisfactory due to scant cellularity. A subsequent biopsy of this gastric lesion showed mucosal amyloid deposition (with no lymphoma or carcinoma) confirmed by Congo red and immunohistochemical staining.
The fourth was the case of an 82‐year‐old woman with diagnosis of linitis plastica by endoscopic examination and EUS. EUS‐FNA of the stomach showed few atypical cells and was unsatisfactory due to scant cellularity. This patient also had simultaneous peritoneal fluid cytology which showed reactive mesothelial cells and no evidence of malignancy. A subsequent gastric biopsy showed acute and chronic inflammation with ulceration; no tumour was seen.
The fifth was the case of a 60‐year‐old woman who presented with abdominal pain. CT scan of the abdomen showed a large 2.6×1.8 cm submucosal gastric mass. EUS‐FNA of this lesion showed debris and epithelial cells and was unsatisfactory due to scant cellularity. A subsequent laparoscopic partial gastrectomy showed GIST.
The sixth was the case of a 50‐year‐old man with history of gastro‐oesophageal reflux disease who presented with a 2×4 cm gastric cardia lesion. EUS‐FNA of this lesion showed only blood and debris on cytology and was unsatisfactory due to scant cellularity. A subsequent biopsy of this lesion showed focal chronic inflammation with no metaplasia/dysplasia.
The calculated sensitivity and specificity of EUS‐FNA of malignant gastric lesions was 91% and 100%, respectively. The positive predictive value was 100%.
Mediastinum
Twelve mediastinal lesions in 11 patients (7 men and 4 women) with a mean age of 62 years (range 35–78 years) were aspirated. The mean size of the lesions was 3.3 cm (range 1.5–6 cm).
Malignant disease was confirmed prior to EUS‐FNA in 4 patients. These included: breast carcinoma (n = 1), oesophageal cancer (n = 1), Hodgkin lymphoma (n = 1), small cell carcinoma of the lung (n = 1), colorectal cancer (n = 1) and non‐Hodgkin lymphoma (n = 1). Two of these patients had malignancy of more than one anatomic site, diagnosed prior to the EUS‐FNA of their present mediastinal lesion.
One patient had a history of breast and oesophageal carcinoma, and the other had a history of colorectal cancer and non‐Hodgkin lymphoma.
The cytological diagnoses were 6 malignant (50%), 3 benign/non‐neoplastic (25%), 2 atypical/suspicious (17%) and 1 non‐diagnostic (8%) case.
The malignant diagnoses included: metastatic adenocarcinoma (n = 2), recurrent non‐Hodgkin lymphoma (n = 1), metastatic poorly differentiated carcinoma (n = 2) and metastatic small cell carcinoma of lung (n = 1).
Two of the 6 malignant aspirates had histopathological follow‐up. In one case the mediastinal mass aspirate showed the presence of adenocarcinoma favouring bronchio‐alveolar type. A subsequent resection of the right upper lobe of the lung showed the presence of a 3.5 cm well differentiated adenocarcinoma, with prominent bronchiolo‐alveolar pattern. In the other case, surgical procedures included two attempts at mediastinal mass biopsy, which were non‐diagnostic due to insufficient tissue. There were no false positive aspirates.
None of the 3 benign/non‐neoplastic cases or 3 atypical/suspicious lesions had histopathological follow‐up.
The calculated sensitivity and specificity of EUS‐FNA of malignant mediastinal lesions was 100%. The positive predictive value was 100%.
Retroperitoneum
Eight patients underwent EUS‐FNA of 8 retroperitoneal lesions (6 men and 2 women). The mean age of the patients was 58 years (range 44–73 years). The mean size of the lesions was 5 cm (range 2–6.5 cm).
Malignant disease was confirmed prior to the procedure in two patients: one patient with pancreatic carcinoma, and the other with colorectal carcinoma and non‐Hodgkin lymphoma. One of these patients had a history of both non‐Hodgkin lymphoma and colorectal carcinoma.
The EUS‐FNA diagnoses were: 1 malignant case (12.5%), 2 benign cases (25%), 1 atypical/suspicious (12.5%) and 4 non‐diagnostic cases (50%).
The one malignant lesion was a non‐Hodgkin lymphoma which was confirmed by flow‐cytometry and subsequent histopathology. There were no false positive aspirates.
Histopathological follow‐up was available in one of the two non‐neoplastic/benign cases. This was the case of a 45‐year‐old man who presented with diffuse mesenteric lymphadenopathy involving the small bowel and retroperitoneum. EUS‐FNA of the retroperitoneal mass showed mature lymphocytes, histiocytes, normal pancreatic cells and hepatocytes. Flow cytometry was non‐diagnostic due to limited cellularity. A subsequent excisional biopsy of the lymph nodes involving the small bowel mesentery and retroperitoneum showed the presence of follicular centre cell lymphoma.
No histopathological follow‐up was available in atypical cases.
One of the 4 non‐diagnostic retroperitoneal aspirates had a surgical pathology follow‐up. This was the case of a 70‐year‐old man who presented with a duodenal/para‐aortic mass and a gastric mass on the lesser curvature on endoscopic examination. EUS‐FNA of this para‐aortic/duodenal mass showed mostly blood on cytology and was unsatisfactory due to scant cellularity. Subsequent biopsy of the gastric mass showed a malignant lymphoma, large B‐cell type with acute and chronic gastritis and a positive Helicobacter pylori stain. The duodenal/para‐aortic mass biopsy also showed focal ulceration and infiltrate consistent with involvement by malignant large cell lymphoma.
The calculated sensitivity and specificity of EUS‐FNA of malignant retroperitoneal lesions was 50% and 100%, respectively. The positive predictive value was 100%.
Lung
Seven lung lesions were aspirated in 7 patients (4 men and 3 women). The mean age of the patients was 73.5 years (range 67–82 years). The mean size of the lesions was 4.15 cm (range 2.3–6 cm).
Two lesions were located in the left upper lobe, one in left lower lobe, one in the left hilum and one in the left supra‐hilum; there was one para‐tracheal mass and one carinal mass.
Malignant disease was confirmed prior to the EUS in 3 patients. This included: oesophageal adenocarcinoma, oro‐pharyngeal squamous cell carcinoma, and squamous cell carcinoma of the lung.
The FNA specimens were diagnosed as: 3 malignant cases (43%), 1 benign/non‐neoplastic (14%), 2 atypical/suspicious (29%) and 1 non‐diagnostic cases (14%).
Malignant lesions included: primary squamous cell carcinoma of the lung (n = 1), poorly differentiated non‐small cell lung cancer (n = 1) and metastatic adenocarcinoma from oesophageal primary (n = 1).
None of the 3 malignant and 1 benign cases had subsequent histological follow‐up. Histopathologic follow‐up was available in both cases diagnosed as atypical/suspicious. One FNA was from a 76‐year‐old woman who presented with a mass in the left upper lobe of lung diagnosed as rare atypical glandular cells, suspicious for but not diagnostic of tumour. A subsequent core biopsy of the lung nodule was suspicious for involvement by adenocarcinoma. A subsequent biopsy of a liver nodule showed adenocarcinoma morphologically suggestive of pancreatico‐biliary origin. The second was the case of a 68‐year‐old man with history of squamous cell carcinoma of oro‐pharynx, status‐post mandible resection and radiotherapy. The patient presented a few years later with a 2.3 cm mass in the left lower lobe of the lung which was diagnosed as highly suspicious for squamous cell carcinoma. Subsequent video‐assisted thoracic surgery and wedge resection of the left lower lobe of the lung established the presence of a 4.5 cm moderately differentiated squamous cell carcinoma in the lung. However, a primary bronchogenic squamous cell carcinoma versus a metastasis from the prior oro‐pharyngeal squamous cell carcinoma could not be distinguished reliably on a histological basis alone.
The calculated sensitivity and specificity of EUS‐FNA of malignant lung lesions was 100% and 100%, respectively. The positive predictive value was 100%.
GI tract
Fifteen patients with GI tract lesions (6 men and 9 women) underwent EUS‐FNA. The mean age of the patients was 58.5 years (range 37–77 years). The mean size of the lesions was 2.48 cm (range 0.8–5 cm). These lesions included: para‐duodenal mass (n = 1), colonic mass at previous anastomosis site (n = 2), peri‐colonic mass (n = 1), rectal mass (n = 6), peri‐rectal mass (n = 3) and pre‐sacral mass (n = 1).
Malignant disease was confirmed prior to EUS in 9 patients: gastric adenocarcinoma (n = 1), colon rectal adenocarcinoma (n = 6), ovarian carcinoma (n = 1) and non‐Hodgkin lymphoma (n = 1).
The FNA diagnoses were malignant/neoplastic in 6 cases (40%), benign/non‐neoplastic in 5 cases (33%), atypical/suspicious in 1 case (7%) and non‐diagnostic in 3 cases (20%).
The malignant/neoplastic diagnoses were: adenocarcinoma (n = 2) (in both cases, it was uncertain whether these were primary or metastatic lesions), neuroendocrine neoplasm (n = 1), metastatic adenocarcinoma (n = 2) (including metastases from gastric and ovarian primaries), and Hodgkin lymphoma (n = 1).
Histopathological follow‐up was available in 4 of the 6 malignant/neoplastic cases. The surgical procedures included endoscopic guided biopsies of duodenal (n = 1) and rectal lesions (n = 3).
There was one false positive aspirate. This was the case of a 37‐year‐old woman with a history of Hodgkin lymphoma, status‐post bone marrow transplant. She presented with a rectal lesion and an enlarged rectal peri‐luminal lymph node, both aspirated during the same procedure. The EUS‐FNA of the rectal lesion showed numerous atypical multinucleated and mono‐nuclear cells in background of lymphocytes, consistent with involvement by Hodgkin disease. The rectal peri‐luminal lymph node FNA showed rare atypical cells, suspicious for but not diagnostic of tumour. A subsequent recto‐sigmoid biopsy showed colonic mucosa with focal acute cryptitis and mild crypt architecture distortion, with no tumour seen. However, the possibility of a deeper submucosal lesion could not be ruled out on histology.
The benign/non‐neoplastic aspirates included 3 rectal lesions, 1 colon anastomotic mass and 1 pre‐sacral mass. Histopathological follow‐up was available in all cases and included 4 biopsies and 1 pre‐sacral mass wall resection.
The 4 EUS‐FNAs from colorectal lesions showed benign and reactive glandular epithelium with mixed inflammatory infiltrate with no evidence of dysplasia or malignancy on cytology. This correlated with their subsequent biopsies showing granulation tissue, acute/chronic inflammation, focal fibrosis, and fibrinopurulent debris without tumour or dysplasia.
The FNA of the pre‐sacral mass showed numerous squamous cells and hyperkeratotic squamous cells admixed with amorphous debris, acute inflammation and faecal material, consistent with a teratoma. A subsequent resection of the pre‐sacral mass wall established the presence of mature teratoma with elements of skin, respiratory mucosa and endocervical type mucosa with surrounding chronic inflammation and fibrosis.
There were no false negative diagnoses.
The one atypical/suspicious aspirate was from a 55‐year‐old man with history of colorectal adenocarcinoma and status‐post low anterior resection and ileostomy. EUS‐FNA of both the colorectal anastomotic site and peri‐rectal lymph node showed rare atypical cells in a background of reactive glandular cells; however, a reactive/reparative process was favoured. A subsequent biopsy of the colon anastomotic site showed focal hyperplasia with no tumour.
The calculated sensitivity and specificity of EUS‐FNA of malignant lesions of the GI tract was 100% and 86%, respectively. The positive predictive value was 83%.
Miscellaneous
Four patients (2 women and 2 men) with miscellaneous lesions underwent EUS‐FNA. The mean age of the patients was 71.2 years (range 49–87 years). The mean size of the lesion was 11 cm (range 10–12 cm). The miscellaneous lesions were: intra‐abdominal mass (n = 3) and umbilical nodule (n = 1). All taspirates resulted in malignant/neoplastic diagnoses on cytology. None of the aspirates were benign/atypical/suspicious or non‐diagnostic.
These neoplastic aspirates were: GIST (n = 2) and adenocarcinoma (n = 2).
Only one of the four malignant aspirates on cytology had adequate histological confirmation. This was the case of a 67‐year‐old woman with history of left breast carcinoma status post‐partial left mastectomy who presented with a 10 cm intra‐abdominal mass. EUS‐FNA of this mass showed a spindle cell neoplasm favouring GIST on cytology, confirmed by immunohistochemical staining. Subsequent laparoscopic excision of the abdominal mass confirmed the presence of GIST involving the stomach and liver on histology.
The calculated sensitivity and specificity of EUS‐FNA of malignant miscellaneous lesions including an intra‐abdominal mass and an umbilical nodule was 100% and 100%, respectively. The positive predictive value was 100%.
Discussion
Several studies have demonstrated the diagnostic value of EUS‐FNA in the work‐up of abdominal lesions, particularly from the pancreas and intra‐abdominal lymph nodes. EUS is superior to other imaging techniques such as CT in lymph node staging of GI and pulmonary malignancies.4,5,6,7,8,12,15,31,32 Our results show high diagnostic sensitivity and specificity comparable to those from previous studies. Cyto‐histological correlates were not available in all cases, including those with a cytological diagnosis of malignancy; however, in most of these cases previous pathological diagnosis and follow‐up clinical data were in concordance with the EUS‐FNA diagnosis.
Most of the samples included in our study were from lymph nodes, followed by hepatobiliary and gastric lesions, as compared with a number of previous studies that analysed samples mostly derived from lymph nodes and pancreas. In this study 122 lymph nodes underwent EUS‐FNA; the sensitivity, specificity, and positive predictive value of EUS‐FNA of lymph nodes were 100%, 99%, and 97%, respectively. There was only one false positive case which was diagnosed as “neuroendocrine tumour”. On the retrospective review the so called “neuroendocrine cells” were most likely contamination from the neighbouring pancreatic tissue inadvertently sampled during EUS‐FNA of the peri‐pancreatic lymph node. Contamination of the EUS‐FNA samples by non‐lesional intestinal surface epithelium and mucin is well‐recognised; it can be mistaken for pancreatic ductal epithelium and mucin producing lesions.7 However, none of these studies have reported accidental sampling of pancreatic endocrine tissue mistaken for neuroendocrine tumour.
Samples from extra‐abdominal sites, such as mediastinum and lung were also included in our study. At present, CT‐guided and transbronchial needle biopsy are more commonly utilised to obtain tissue samples from lung and mediastinum as compared to EUS‐FNA. It has been shown that EUS‐FNA is comparable and even superior in some instances in obtaining tissue samples from mediastinum and lung to establish a diagnosis of primary and metastatic neoplasms.33 Even though the histopathological follow‐up was limited in the present study, the sensitivity and specificity of EUS‐FNA in the diagnosis of malignant lesions of mediastinum and lung were 100%. These results are similar to those reported by others.11,12,19,26,33,34,35,36,37,38,39
The calculated sensitivity, specificity, and positive predictive value of EUS‐FNA of GI lesions were 100%, 86%, and 83% respectively, compared with 89% and 88% reported by Vander Noot et al.40 There was one false positive case in which the reactive and reparative changes from a case of acute cryptitis were mistaken for involvement by patients with known Hodgkin's disease. It is well established that the reactive and reparative changes involving the GI tract could be mistaken for neoplastic changes due to marked reactive/reparative atypia of either the glandular epithelium or the inflammatory infiltrate.41,42 On a retrospective review the cells mistaken for Reed–Sternberg cells could be immunoblasts from the germinal centre rather than tumour cells. However, the negative biopsies showing inflammatory changes only were limited to the superficial layers of the bowel wall, whereas the EUS‐FNA sample was from all the layers of the bowel wall. There was no further follow‐up available to confirm the surgical pathology diagnosis. There was one false negative case diagnosed as GIST on partial gastrectomy. The difficulty in the assessment of submucosal lesions by aspiration cytology, particularly GIST, has been described. Similarly smooth muscle from the GI tract wall could be mistaken for GIST or smooth muscle tumours.7 However, immunostains for smooth muscle, c‐kit (CD117) and CD34 are usually helpful to arrive at a correct diagnosis.
EUS‐FNA operating characteristics were comparable among the different groups of lesions included in our study except for specimens obtained from peri‐pancreatic lesions and retroperitoneum. Sensitivity was 60% in the former and 50% in the latter group; however, this may not be a true reflection of the EUS‐FNA test due to the limited number of cases in both groups. In this series, EUS‐FNA not only provided sufficient material for an accurate cytological diagnosis, it also allowed for staging of many malignant lesions without the need for more complex diagnostic procedures. However, as with other procedures a successful EUS‐FNA requires a skilled clinician and an experienced cytopathology team to provide on‐site evaluation. On‐site evaluation of cytology specimens have proven to be very useful in markedly reducing the sampling errors, non‐diagnostic cases due to limited cellularity, and effective triage of cases requiring special studies (flow cytometric analysis of lymphoma samples).43,44,45 One study of on‐site evaluation of FNA specimens from our institution has shown a cost benefit of $404 000 (£200 000; €300 000) per year by reducing repeat FNA procedures due to non‐diagnostic specimens.45 In our study all EUS‐FNA procedure were performed by a team of gastroenterologists experienced in performing EUS‐FNA, and all specimens were evaluated on‐site for adequacy and diagnosis by an experienced cytopathologist. The non‐diagnostic rate was 22% (53/246) and half of the specimens were EUS‐FNA of lymph nodes. This high rate in this group could be explained on the basis of location and small size of lesions (size range 0.5–4.0 cm, mean 1.83 cm).
In conclusion, our institutional experience confirms that EUS‐FNA is a safe and reliable method that provides the cytopathologist with adequate specimens for a cytopathological diagnosis with high sensitivity and specificity. EUS‐FNA of extra‐pancreatic lesions not only provides for accurate cytological diagnosis but also allows for preoperative tumour staging, therefore influencing the therapeutic management of these lesions.
Take‐home message
Endoscopic ultrasound guided fine needle aspiration is an effective method with high sensitivity, specificity, and positive predictive value in the diagnosis of non‐pancreatic lesions.
Abbreviations
EUS - endoscopic ultrasound
FNA - fine needle aspiration
GI - gastrointestinal
GIST - gastrointestinal stromal tumour
Footnotes
Competing interests: None declared.
References
- 1.Chang K J. Endoscopic ultrasound (EUS)‐guided fine needle aspiration (FNA) in the USA. Endoscopy 199830(Suppl 1)A159–A160. [DOI] [PubMed] [Google Scholar]
- 2.Hawes R H. Indications for EUS‐directed FNA. Endoscopy 199830(Suppl 1)A155–A157. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani S, Ohhashi K, Yamao K.et al [Usefulness for choice of treatment by endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) cytology of ascites—report of two cases]. Nippon Shokakibyo Gakkai Zasshi 1998951047–1051. [PubMed] [Google Scholar]
- 4.Jhala D, Jhala N C. Endoscopic ultrasound guided fine needle aspiration of the pancreas. Adv Exp Med Biol 200556391–103. [DOI] [PubMed] [Google Scholar]
- 5.Jhala N C, Jhala D, Eloubeidi M A.et al Endoscopic ultrasound‐guided fine‐needle aspiration biopsy of the adrenal glands: analysis of 24 patients. Cancer 2004102308–314. [DOI] [PubMed] [Google Scholar]
- 6.Jhala N C, Jhala D, Eltoum I.et al Endoscopic ultrasound‐guided fine‐needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer 2004102239–246. [DOI] [PubMed] [Google Scholar]
- 7.Jhala N C, Jhala D N, Chhieng D C.et al Endoscopic ultrasound‐guided fine‐needle aspiration. A cytopathologist's perspective. Am J Clin Pathol 2003120351–367. [DOI] [PubMed] [Google Scholar]
- 8.Bentz J S, Kochman M L, Faigel D O.et al Endoscopic ultrasound‐guided real‐time fine‐needle aspiration: clinicopathologic features of 60 patients. Diagn Cytopathol 19981898–109. [DOI] [PubMed] [Google Scholar]
- 9.Faigel D O, Ginsberg G G, Bentz J S.et al Endoscopic ultrasound‐guided real‐time fine‐needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol 1997151439–1443. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad N A, Kochman M L, Lewis J D.et al Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol 2001963295–3300. [DOI] [PubMed] [Google Scholar]
- 11.Caddy G, Conron M, Wright G.et al The accuracy of EUS‐FNA in assessing mediastinal lymphadenopathy and staging patients with NSCLC. Eur Respir J 200525410–415. [DOI] [PubMed] [Google Scholar]
- 12.Chhieng D C, Jhala D, Jhala N.et al Endoscopic ultrasound‐guided fine‐needle aspiration biopsy: a study of 103 cases. Cancer 200296232–239. [DOI] [PubMed] [Google Scholar]
- 13.Crowe D R, Eloubeidi M A, Chhieng D C.et al Fine‐needle aspiration biopsy of hepatic lesions: computerized tomographic‐guided versus endoscopic ultrasound‐guided FNA. Cancer 2006108180–185. [DOI] [PubMed] [Google Scholar]
- 14.Binmoeller K F. EUS instruments for FNA. Endoscopy 199830(Suppl 1)A158. [DOI] [PubMed] [Google Scholar]
- 15.Eloubeidi M A, Seewald S, Tamhane A.et al EUS‐guided FNA of the left adrenal gland in patients with thoracic or GI malignancies. Gastrointest Endosc 200459627–633. [DOI] [PubMed] [Google Scholar]
- 16.Eloubeidi M A, Tamhane A. EUS‐guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc 200561700–708. [DOI] [PubMed] [Google Scholar]
- 17.Yamao K, Ohashi K, Mizutani S.et al Endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) for the diagnosis of digestive diseases. Endoscopy 199830(Suppl 1)A176–A178. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen P, Feng J C, Chang K J. Endoscopic ultrasound (EUS) and EUS‐guided fine‐needle aspiration (FNA) of liver lesions. Gastrointest Endosc 199950357–361. [DOI] [PubMed] [Google Scholar]
- 19.Fritscher‐Ravens A, Sriram P V, Bobrowski C.et al Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS‐FNA‐based differential cytodiagnosis in 153 patients. Am J Gastroenterol 2000952278–2284. [DOI] [PubMed] [Google Scholar]
- 20.Annema J T, Veselic M, Versteegh M I.et al Mediastinitis caused by EUS‐FNA of a bronchogenic cyst. Endoscopy 200335791–793. [DOI] [PubMed] [Google Scholar]
- 21.Hollerbach S, Reiser M, Topalidis T.et al Diagnosis of hepatocellular carcinoma (HCC) in a high‐risk patient by using transgastric EUS‐guided fine‐needle biopsy (EUS‐FNA). Z Gastroenterol 200341995–998. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson B C, Pitman M B, Brugge W R. EUS‐guided FNA for the diagnosis of gallbladder masses. Gastrointest Endosc 200357251–254. [DOI] [PubMed] [Google Scholar]
- 23.Okada N, Hirooka Y, Itoh A.et al Retroperitoneal neurilemoma diagnosed by EUS‐guided FNA. Gastrointest Endosc 200357790–792. [DOI] [PubMed] [Google Scholar]
- 24.Sangha S, Gergeos F, Freter R.et al Diagnosis of ovarian cancer metastatic to the stomach by EUS‐guided FNA. Gastrointest Endosc 200358933–935. [DOI] [PubMed] [Google Scholar]
- 25.Savides T J. EUS FNA staging of esophageal cancer. Gastroenterology 20031251883–1886. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez L V, Mishra G, George S.et al A descriptive analysis of EUS‐FNA for mediastinal lymphadenopathy: an emphasis on clinical impact and false negative results. Am J Gastroenterol 200499249–254. [DOI] [PubMed] [Google Scholar]
- 27.Jovanovic I, Knezevic S, Micev M.et al EUS mini probes in diagnosis of cystic dystrophy of duodenal wall in heterotopic pancreas: a case report. World J Gastroenterol 2004102609–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al‐Haddad M, Wallace M B. EUS‐FNA and biomarkers for the staging of non‐small cell lung cancer. Endoscopy 200638(Suppl 1)S114–S117. [DOI] [PubMed] [Google Scholar]
- 29.Sawhney M S, Kratzke R A, Lederle F A.et al EUS‐guided FNA for the diagnosis of advanced lung cancer. Gastrointest Endosc 200663959–965. [DOI] [PubMed] [Google Scholar]
- 30.Wang K P. EUS‐guided FNA immediately after unrevealing transbronchial needle aspiration in the evaluation of mediastinal lymphadenopathy: a prospective study. Gastrointest Endosc 200664468–470. [DOI] [PubMed] [Google Scholar]
- 31.Williams D B, Sahai A V, Aabakken L.et al Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 199944720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eloubeidi M A, Varadarajulu S, El‐Galley R.et al EUS‐guided FNA for the diagnosis of recurrent bladder cancer through the ileal conduit: a novel approach. Gastrointest Endosc 200664450–453. [DOI] [PubMed] [Google Scholar]
- 33.Vilmann P, Krasnik M, Larsen S S.et al Transesophageal endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) and endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 200537833–839. [DOI] [PubMed] [Google Scholar]
- 34.Sobel J M, Lai R, Mallery S.et al The utility of EUS‐guided FNA in the diagnosis of metastatic breast cancer to the esophagus and the mediastinum. Gastrointest Endosc 200561416–420. [DOI] [PubMed] [Google Scholar]
- 35.Sudhoff T, Hollerbach S, Wilhelms I.et al [Clinical utility of EUS‐FNA in upper gastrointestinal and mediastinal disease]. Dtsch Med Wochenschr 20041292227–2232. [DOI] [PubMed] [Google Scholar]
- 36.Varadarajulu S, Hoffman B J, Hawes R H.et al EUS‐guided FNA of lung masses adjacent to or abutting the esophagus after unrevealing CT‐guided biopsy or bronchoscopy. Gastrointest Endosc 200460293–297. [DOI] [PubMed] [Google Scholar]
- 37.Annema J T, Veselic M, Versteegh M I.et al Mediastinal restaging: EUS‐FNA offers a new perspective. Lung Cancer 200342311–318. [DOI] [PubMed] [Google Scholar]
- 38.Dewitt J, Ghorai S, Kahi C.et al EUS‐FNA of recurrent postoperative extraluminal and metastatic malignancy. Gastrointest Endosc 200358542–548. [DOI] [PubMed] [Google Scholar]
- 39.Chhieng D C, Lin O, Moran C A.et al Fine‐needle aspiration biopsy of nonteratomatous germ cell tumors of the mediastinum. Am J Clin Pathol 2002118418–424. [DOI] [PubMed] [Google Scholar]
- 40.Vander Noot M R, 3rd, Eloubeidi M A, Chen V K.et al Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound‐guided fine‐needle aspiration biopsy. Cancer 2004102157–163. [DOI] [PubMed] [Google Scholar]
- 41.Green P H, Gold R P, Marboe C C.et al Chronic erosive gastritis: clinical, diagnostic, and pathological features in nine patients. Am J Gastroenterol 198277543–547. [PubMed] [Google Scholar]
- 42.Weidner N, Smith J G, LaVanway J M. Peptic ulceration with marked epithelial atypia following hepatic arterial infusion chemotherapy. A lesion initially misinterpreted as carcinoma. Am J Surg Pathol 19837261–268. [DOI] [PubMed] [Google Scholar]
- 43.Eloubeidi M A, Tamhane A, Jhala N.et al Agreement between rapid onsite and final cytologic interpretations of EUS‐guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol 20061012841–2847. [DOI] [PubMed] [Google Scholar]
- 44.Levy M J, Jondal M L, Clain J.et al Preliminary experience with an EUS‐guided trucut biopsy needle compared with EUS‐guided FNA. Gastrointest Endosc 200357101–106. [DOI] [PubMed] [Google Scholar]
- 45.Nasuti J F, Gupta P K, Baloch Z W. Diagnostic value and cost‐effectiveness of on‐site evaluation of fine‐needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol 2002271–4. [DOI] [PubMed] [Google Scholar]