A 5‐year‐old boy was referred with a history of headache, vomiting, abnormal body movements and altered sensorium for 2 days. The past and family history was not significant. Neurological examination revealed features of raised intracranial tension—that is, intermittent tonic posturing of the body, asymmetric non‐reacting pupils and blurring of the nasal margin of the fundus. In addition, he had increased tone in the right upper and lower limbs and upgoing plantar reflex. The child was intubated and treatment with mannitol and phenytoin was commenced. The provisional diagnosis was acute febrile encephalopathy; ceftriaxone and acyclovir were commenced. CT scan showed a mass lesion (7×5.5×4 cm) in the left parieto‐temporal region with haemorrhage inside it. There was midline shift and the lesion was abutting the trigone of the left lateral ventricle (fig 1). He underwent surgery and the lesion was examined by histopathology.
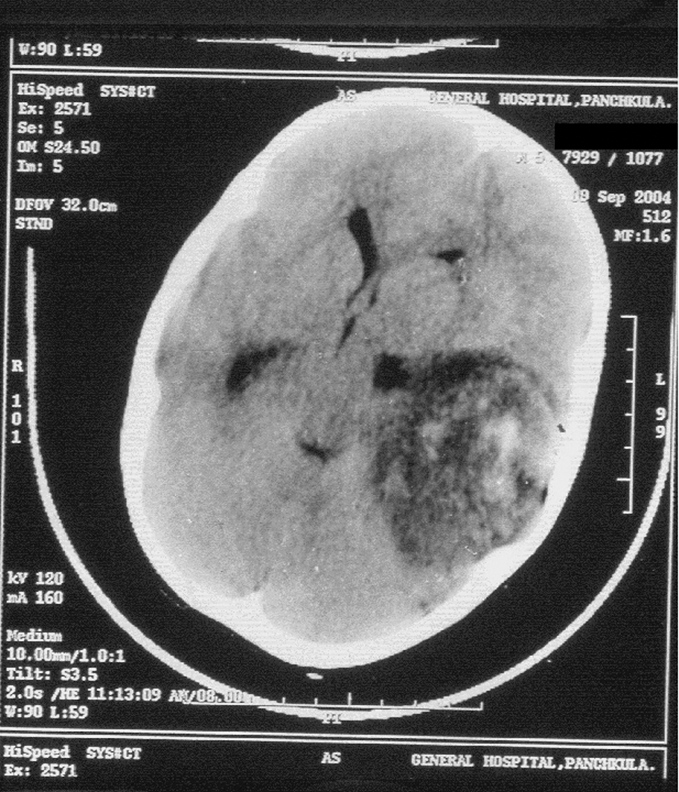
Figure 1 CT scan showing a large mass lesion in the left parieto‐temporal region with midline shift.
Light microscopy revealed multiple fragments of a tumour admixed with blood clots. The tumour cells were arranged in cords and small clusters and were lying in a mucinous vacuolated background. The cells were relatively uniform, oval to polygonal, and had abundant eosinophilic cytoplasm (fig 2). No mitotic figures were seen. A few fragments showed limited glial differentiation in the form of coarsely fibrillar processes. No histological features of meningioma such as whorls, psammoma bodies or nuclear pseudoinclusions were identified. For immunohistochemistry, sections were treated with monoclonal antibodies (Dako Corp., Carpinteria, California, USA). Staining was carried out for glial fibrillary acidic protein (GFAP) (1:100), vimentin (1:200), S‐100 (1:400), CD 34 (1:100), cytokeratin (CK) (1:150) and epithelial membrane antigen (EMA) (1:80) using the peroxidase–antiperoxidase method. The tumour cells were positive for GFAP, S‐100 and vimentin, focally positive for CD 34 but negative for CK and EMA. A sample for electron microscopic examination was taken from the paraffin embedded tissue, therefore the ultrastructural findings could not be defined fully. However, the tumour cells showed cytoplasmic intermediate filaments, intermediate junctions and focal basal lamina. There were no cilia, complex interdigitations of cell membrane or well‐formed desmosomes. Based on the histomorphology, immunohistochemistry and ultrastructural findings, unusual glioma with extensive myxoid change resembling chordoid glioma was diagnosed. The child had a cardiac arrest 2 days after the surgery and could not be revived.
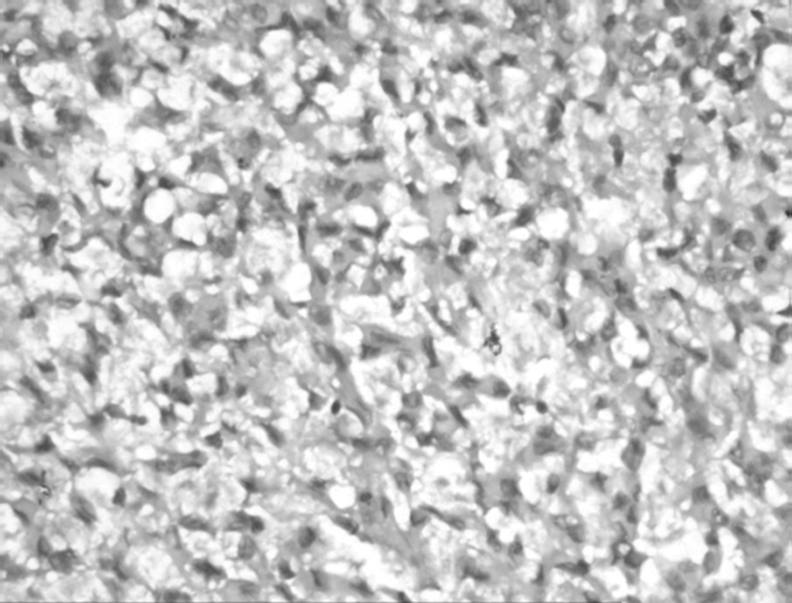
Figure 2 Photomicrograph of the tumour showing cords of tumour cells in mucinous vacuolated background (H&E, original magnification ×20).
Discussion
The features of the index case resembled chordoid glioma (CG) of the third ventricle, which was described in 1998 by Brat et al as a novel clinicopathological entity based on a series of eight cases.1 Since the initial description, only 37 cases have been documented in the literature that all highlight the unique clinical, neuroradiological, and pathological characteristics of this lesion.2 The immunohistochemical and ultrastructural studies indicate that CGs are glial in nature; they are placed in the category of glial tumours of uncertain origin in the latest World Health Organization classification.3,4 CGs usually occur in adult patients (mean age 44.9 years, range 24–70 years), with a 1.7:1 female predominance.5 To the best of our knowledge, only one case in a child (12 years) has been described previously.6
Chordoid glioma shows a strikingly stereotypical anatomic localization in the hypothalamic/suprasellar/third ventricular region.5 However, an unusual and multicystic component extending within the right temporal lobe and sella turcica was mentioned on MRI in only one of the cases by Brat et al.1 In another exceptional case, the autopsy findings confirmed a tumour connection not only with the roof and floor of third ventricle but also with the right thalamus as well as a tumour extension within the hypothalamus and chiasmatic cistern.7 CG has never been reported in cerebral hemispheres. In the index case, the tumour is located in the left parieto‐temporal region and was abutting the trigone of the left lateral ventricle.
The microscopic findings were typical for chordoid glioma but there was no lymphoplasmacytic infiltrate. Strong lymphoplasmacytic infiltrate with numerous Russell bodies is a feature in CG, which was lacking in our case. However, an occasional case lacking lymphoplasmacytic infiltrate and Russell bodies has been described in the literature.1 Another feature that is usually present is reactive astrocytes and Rosenthal fibres in the adjacent non‐neoplastic tissue.5 In the present case, no adjacent non‐neoplastic tissue was included. However, the histomorphology, GFAP positivity and ultrastructural findings led us to make the diagnosis of unusual glioma with extensive myxoid change resembling CG.
The strikingly “chordoid” appearance of the neoplasms, with their eosinophilic clustered tumour cells in a blue mucinous matrix is distinctive among other regional tumours including pituitary adenoma, craniopharyngioma, pilocytic astrocytoma, and meningioma. The presence of GFAP reactivity and the absence of synaptophysin staining are inconsistent with pituitary adenoma. Pilocytic astrocytoma and craniopharyngioma bear even less of a morphological resemblance to chordoid glioma. Although there are histological similarities between chordoid gliomas and chordoid meningioma such as clustering of epithelioid cells, these meningiomas are typically dura based, and have a more prominent lymphoplasmacytic component often featuring germinal centres.4 In the index case, the lesion was not dura based. The other features against chordoid meningioma were GFAP positivity, EMA negativity and absence of complex interdigitations of cell membrane or well formed desmosomes. Also, chordoid meningioma invariably contains foci of identifiable meningioma.8 Another differential diagnosis is chordoma, which usually has a close relationship with the bone, but it is possible to find it in the intradural space without any apparent bone connection.9,10 A strong inflammatory infiltrate can be seen in the stroma, but physaliphora cells are usually prominent. Moreover, intense immunoreactivity for CK, especially CK 8 and 1810 and mitochondria–rough endoplasmic reticulum complexes on ultrastructural examination confirms the diagnosis of chordoma.11
Reifenberger et al showed that the genetic anomalies characteristic of astrocytomas, meningiomas and chordomas are not features of CG of the third ventricle.12 Ultrastructural study of chordoid gliomas lends support for their glial derivation. The presence of focal basal lamina formation and of microvilli in most cases suggests the possibility of ependymal derivation. The more definitive ultrastructural features of ependymoma, such as cilia and desmosomal junctions are usually lacking.4 However, an occasional case with few tumour cells showing isolated abnormal cilia in the vicinity of the nucleus has recently been reported.5 In the present case, based on negativity for ependymal features (EMA negativity and lack of microvillous structures on electron microscopy), GFAP positivity and a large number of intermediate filaments on ultrastructure, the possible origin of the tumour is astrocytic.
In summary, the index case is a 5‐year‐old boy with an unusual extraventricular glioma with extensive myxoid change resembling chordoid glioma, occurring in left parieto‐temporal region.
Footnotes
Competing interests: None.
References
- 1.Brat D J, Scheithauer B W, Staugaitis S M.et al Third ventricular chordoid glioma: a distinct clinicopathologic entity. J Neuropathol Exp Neurol 199857283–290. [DOI] [PubMed] [Google Scholar]
- 2.Kurian K M, Summers D M, Statham P F X.et al Third ventricular chordoid glioma: clinicopathological study of two cases with evidence for a poor clinical outcome despite low grade histological features. Neuropathol Applied Neurobiol 200531354–361. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Louis D N, Scheithauer B W.et al The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 200261215–225. [DOI] [PubMed] [Google Scholar]
- 4.Brat D J, Scheithauer B W, Cortez S C.et al Chordoid glioma of the third ventricle. In: Kleihues P, Cavenee WK, eds. Pathology and genetics: tumors of the nervous system. World Health Organization Classification. Lyon: International Agency for Research on Cancer, 200090–91.
- 5.Pasquier B, Peoc'h M, Morrison A L.et al Chordoid glioma of the third ventricle. Am J Surg Pathol 2002261330–1342. [DOI] [PubMed] [Google Scholar]
- 6.Castellano‐Sanchez A A, Schemankewitz E, Mazewski C.et al Pediatric chordoid glioma with chondroid metaplasia. Pediatr Dev Pathol 20014564–567. [DOI] [PubMed] [Google Scholar]
- 7.Vajtai I, Varga Z, Scheithauer B W.et al Chordoid glioma of the third ventricle: confirmatory report of a new entity. Hum Pathol 199930723–726. [DOI] [PubMed] [Google Scholar]
- 8.Louis D N, Scheithauer B W, Budka H.et al Meningiomas. In: Kleihues P, Cavenee WK, eds. Pathology and genetics: tumors of the nervous system. World Health Organization Classification. Lyon: International Agency for Research on Cancer, 2000176–184.
- 9.Nishigaya K, Kaneko M, Ohashi Y.et al Intradural retroclival chordoma without bone involvement: no tumour regrowth 5 years after operation. Case report. J Neurosurg 199888764–768. [DOI] [PubMed] [Google Scholar]
- 10.Vaz R M, Pereira J C, Ramos U.et al Intradural cervical chordoma without bone involvement. Case report. J Neurosurg 199582650–653. [DOI] [PubMed] [Google Scholar]
- 11.Carstens P E B. Chordoid tumors; a light, electron microscopic, and immunohistochemical study. Ultrastruct Pathol 199519291–295. [DOI] [PubMed] [Google Scholar]
- 12.Reifenberger G, Weber T, Weber R G.et al Chordoid glioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel entity. Brain Pathol 19999617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]


