Endothelial dystrophies produce characteristic morphological and functional abnormalities of the cornea. The most prevalent is Fuchs' endothelial corneal dystrophy (FECD), which is characterized by bilateral primary cornea guttata and a reduced endothelial cell density that can result in corneal oedema, discomfort, and blurred vision. Histology shows a thickened Descemet's membrane with focal posterior excrescences and endothelial cell loss. The onset of FECD is typically in the fifth decade of life,1 but an early‐onset variant has been described that shows phenotypic differences from the more common late‐onset disease.2,3 A genome‐wide search of a three‐generation family with early‐onset FECD identified a locus on chromosome 1p34.3–p32.2 Within this locus a pathogenic mutation p.Q455K was found in the COL8A2 gene in this and two additional pedigrees.2 Gottsch et al4 recently reported a novel mutation p.L450W in a separate family with early‐onset FECD.
Case report
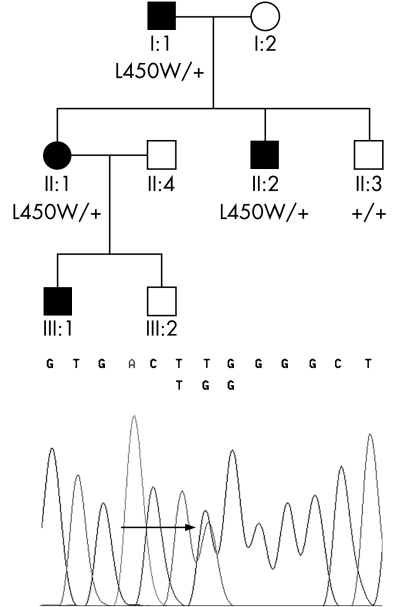
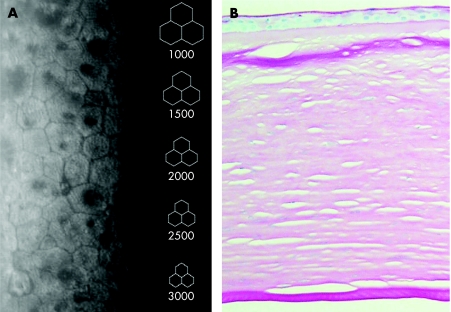
A white British family with early‐onset FECD (fig 1) was identified. Patient I:1 (79 years) had been told he exhibited endothelial pathology when he was 23 years old. At the age of 75 years he had a left penetrating keratoplasty with cataract extraction and intraocular lens implantation. Cornea guttata were present in the right eye. Patient II:1 (53 years) experienced visual deterioration in her mid‐twenties as a result of bilateral corneal oedema. A right penetrating graft was performed at age 34 years and a left penetrating keratoplasty was performed at age 41 years. Patient II:2 (55 years) was asymptomatic, but non‐contact specular microscopy showed endothelial pleomorphism and cornea guttata located both centrally and within the borders of endothelial cells (fig 2A). Patient III:1 (18 years) was documented to have endothelial changes at 9 years of age but still has a corrected acuity of 6/6. Histology of the cornea from patient I:1 showed thickening of Descemet's membrane without cornea guttata (fig 2B).
Figure 1 Fuchs' endothelial corneal dystrophy pedigree and mutation segregation. Affected patients are shown as filled symbols. Heterozygous individuals (I:1, II:1 and II:2) carry the c.1349 T>G transversion mutation resulting in a p.L450W change in the COL8A2 gene, which is indicated by an arrow in the electropherogram. This change was not seen in the unaffected individual (II:3).
Figure 2 Morphological structure of early‐onset Fuchs' endothelial corneal dystrophy corneas (A) specular endothelial microscopy of individual II:2. There is cellular pleomorphism and multiple non‐reflecting areas. (B) Histology of cornea of individual I:1. There is a thickened Descemet's membrane but without posterior excrescences.
Using eight primer pairs the coding region of the COL8A2 gene was sequenced and a previously reported heterozygous point mutation leading to p.L450W substitution4 was identified in family member I:1. The mutation was subsequently confirmed by direct sequencing in two other affected family members (fig 1). Individual III:1 has not yet been tested for this change.
Comment
Mutations in the COL8A2 gene account for only a small proportion (less than 5%)2 of late‐onset FECD, but are associated with early onset disease.2,4 We describe the phenotype of early‐onset FECD in a white British family, which is caused by a point mutation (resulting in p.L450W substitution) in COL8A2. The age of onset, slit‐lamp biomicroscopy findings, and endothelial imaging are similar to the phenotype of a family originally described by Magovern et al.3 in 1979, in which the p.L450W change was subsequently reported.4,5 The phenotype, the early age of onset with endothelial changes detected as early as the first decade, the presence of apparently intracellular guttae on specular microscopy, and the absence of excrescences on Descemet's membrane on histology are the noteworthy clinical features of the present pedigree. The relationship between the early and late‐onset variants of FECD is, at present, uncertain.6
In conclusion, the identification of the p.L450W substitution in a second pedigree suggests that codon 450 in the COL8A2 sequence might be a mutation hotspot. The possibility also exists that the two families share a common ancestor. Unfortunately, no information on other first‐degree relatives of patient I:1 is available.
Acknowledgements
The authors are grateful to the family members who kindly agreed to take part in this study. The authors also thank Dr George Meligonis for performing cornea histology. The study was supported by the Special Trustees of Moorfields Eye Hospital.
Local ethics approval was obtained for this study.
Footnotes
Competing interests: None declared.
References
- 1.Krachmer J H, Purcell J J., Jr Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol 1978962036–2039. [DOI] [PubMed] [Google Scholar]
- 2.Biswas S, Munier F L, Yardley J.et al Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet 2001102415–2423. [DOI] [PubMed] [Google Scholar]
- 3.Magovern M, Beauchamp G R, McTigue J W.et al Inheritance of Fuchs' combined dystrophy. Ophthalmology 1979861897–1923. [DOI] [PubMed] [Google Scholar]
- 4.Gottsch J D, Sundin O H, Liu S H.et al Inheritance of a novel COL8A2 mutation defines a distinct early‐onset subtype of fuchs corneal dystrophy. Invest Ophthalmol Vis Sci 2005461934–1939. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch J D, Zhang C, Sundin O H.et al Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci 2005464504–4511. [DOI] [PubMed] [Google Scholar]
- 6.Sundin O H, Jun A S, Broman K W.et al Linkage of late‐onset Fuchs corneal dystrophy to a novel locus at 13pTel–13q12.13. Invest Ophthalmol Vis Sci 200647140–145. [DOI] [PubMed] [Google Scholar]