Abstract
Aims
The aim of this study was to assess the efficacy of currently available topical drugs for vernal keratoconjunctivitis (VKC) through a meta‐analysis of randomised clinical trials (RCTs).
Methods
Twenty‐seven RCTs (n = 2184 eyes) that had evaluated the efficacy of topical drugs for the treatment of VKC were selected according to the set criteria; 10 of these trials were suitable for statistical analysis and were enrolled in the meta‐analysis. Articles published up to December 2005 were identified from the following data sources: Medline, Embase, Lilacs, the Cochrane Controlled Trials Register, and references from relevant articles. Articles in any language published with an English abstract, were screened, and those selected for inclusion were written in English, French, German, Italian, Portuguese or Spanish. The quality of the trials was assessed by the Delphi list. Statistical analysis was performed using STATA® software.
Results
A significant improvement in all signs and symptoms, except photophobia, was observed after topical treatment for active VKC, independent of the type of treatment. Comparison of the efficacy of different drugs was not possible due to a lack of standardised criteria among studies.
Conclusion
The currently available topical drugs are effective in treating acute phases of VKC. However, there is a lack of evidence to support the recommendation of one specific type of medication for treating this disorder. There is a need for standard criteria to assess diagnosis and therapy based on severity. There is also a need for RCTs assessing long‐term effects of single drugs to control the disease and to prevent complications.
Vernal keratoconjunctivitis (VKC) is a recurrent bilateral chronic allergic inflammatory disease of the ocular surface affecting mainly young males in the first decade of life. Diagnosis is based on signs and symptoms including itching, photophobia, sticky mucous discharge, giant papillae on the upper tarsal conjunctiva or at the limbus, superficial keratopathy and corneal shield ulcer. An immunopathogenic mechanism has been proposed for this disease on the basis of personal or familial history of atopy, increased serum levels of total and specific IgE, the response to antiallergic therapy and the presence of several immune cells and mediators in the conjunctiva.1,2,3
At present, the exact pathogenic mechanism has not been completely identified. In spite of its generally benign and self‐limited presentation, therapeutic measures are required to control signs and symptoms of the disease and to avoid the longstanding permanent inflammatory sequelae that may lead to fibrovascular reaction, new collagen deposition, tissue remodelling and permanent visual damage.1,2,3
Several reports indicate that topical anti‐inflammatory and antiallergic eye‐drops are the mainstay of treatment for VKC, but a gold‐standard treatment has not yet been established for this disease.4,5
In the present report, we systematically reviewed RCTs and conducted a meta‐analysis of the combined results on all available topical drugs for VKC, including antihistamines, mast cell stabilisers, NSAIDs, corticosteroids, immunomodulators and antimitotics, to confirm that topical therapy is an effective treatment in patients with VKC and to establish which therapeutic regimen is most suitable for this condition. To obtain satisfactory homogeneity, we adopted strict eligibility criteria.
Materials and methods
Literature search
Six observers, divided into three groups of two, independently performed a literature search of all publication years up to December 2005. The articles were identified through a computerised search in the Cochrane Controlled Trial Register, CENTRAL/CCTR (which contains the Cochrane Eyes and Vision Group trials register) on the Cochrane Library, MEDLINE, EMBASE and LILACS (Latin American and Caribbean Health Sciences Literature Database). The search strategy was used to identify randomised clinical trials, as recommended by the Cochrane Collaboration.6
Keywords/Search terms for disease were: vernal and keratoconj*, explode vernal keratoconjunctivitis/ all subheadings, vkc/ all subheadings. Keywords/Search terms for medications were: antihistamine, antazoline, azelastine, levocabastine, emedastine, pheniramine; mast cell stabiliser, sodium cromoglycate, lodoxamide, nedocrimil, spaglumic acid; olopatadine, ketotifen, epinastine; corticosteroid, betamethasone, clobethasone, dexamethasone, fluorometholone, hydrocortisone, loteprednol etabonate, medrysone, prednisolone, rimexolone; NSAID, fluorbiprofen, ketorolac, ibuprofen, indomethacin, pranoprofen; ciclosporin; antimitotic, mitomycin‐C.
In addition, linked references in all relevant articles as well as the reviewer's personal collections of articles on vernal keratoconjunctivitis were searched. The search resulted in a total of 333 abstracts.
Inclusion and exclusion criteria
Articles potentially eligible for inclusion in this meta‐analysis were double‐masked randomised clinical trials on topical therapy for vernal keratoconjunctivitis published up to December 2005, written in English, French, German, Italian, Portuguese or Spanish. Additional inclusion criteria for the trials were: follow‐up of at least 2 weeks and adequate wash‐out from previous treatment. Articles were excluded if they did not satisfy one or more inclusion criteria, or if they were irretrievable after performing all available search strategies, including a request to authors and editors.
The article's eligibility was initially determined by evaluating the titles, abstracts and MeSH (medical subject headings). Four observers divided into two groups of two examined all the retrieved 333 abstracts to consider their eligibility. After matching the decisions of the two groups, 285 articles were excluded because they were either not on topical treatments or related to different kinds of ocular and/or systemic allergy. The remaining 48 complete articles were obtained and printed to identify whether they were randomised clinical trials. Four articles were excluded because they did not match this criterion.
To select the trials to be included in this meta‐analysis, the remaining 44 potentially eligible trials were distributed to four researchers divided into two groups of two. The observers were blinded to the names of the authors and institutions, the name of the journals, the sources of funding, and the sponsors of the studies. The observers of each group were also blinded to the decisions of the other group, and trial selection was matched between them. Seventeen trials were excluded because they did not match one or more inclusion criteria (not double‐masked; follow‐up shorter than 2 weeks; no/inadequate wash‐out from previous treatment/s).
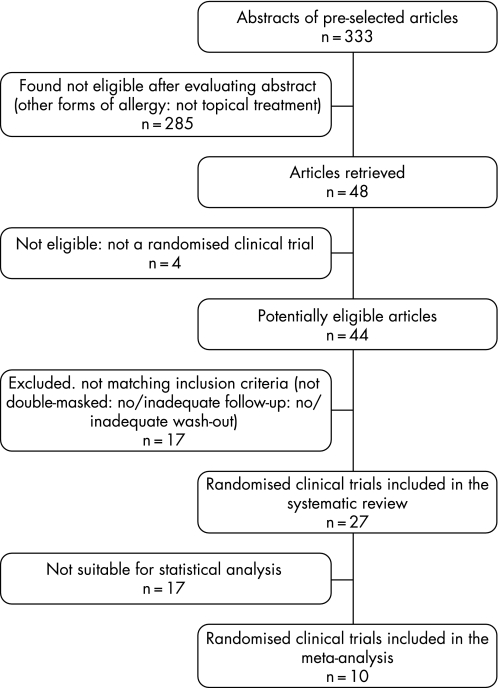
All the remaining 27 trials were included in the systematic review, while only 10 of them were included in the meta‐analysis because they presented comparable data suitable for quantitative statistical analysis (fig 1).
Figure 1 Flow chart demonstrating the selection process for study inclusion in meta‐analysis.
Data extraction
Data were extracted from each article using a standardised form.
Quality assessment
Methodological quality was evaluated using the Delphi list.7 Each item in this quality list had the same weight. For each publication, a quality score was calculated, where “YES” was scored as 1 point for a certain quality item and “NO” and “DO NOT KNOW” were scored as 0 points.
Outcome measures
The mean change from baseline to the end of treatment was identified for the following parameters: itching, tearing, photophobia, hyperaemia, tarsal papillae, limbal disease and corneal involvement.
Statistical analysis
STATA® software8 was used to analyse the data. When two or more studies reported comparable results for the same outcome, those results were presented in an analysis table. The forest plots used were generated by STATA® software. In a forest plot, the results of combined studies are shown as squares centred on the standardised mean difference for the specified outcome for each study. The horizontal line through the square indicates the 95% CI for the mean. At the bottom of each plot, there is a diamond, the centre and extent of which indicate the mean and CI of the pooled results from all the studies. If the diamond is clear of the central vertical line of no effect, the data are considered significant at the stated level (in this study, 5%). The outcomes in this meta‐analysis have all been reported such that a diamond to the left of the central line indicates an effect in favour of topical treatment for VKC.
We tested the heterogeneity between studies using the χ2 test, with significant heterogeneity (p<.05) precluding meta‐analysis.
Results
Description of studies
The 27 studies included in the systematic review dated from 1972 to 2003; 22 of these were published in ophthalmic journals.9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 Eleven trials were performed in Europe, seven in Asia, five in America and three in Africa, and one was a multicentred study conducted in Europe and America. There were seven multicentred studies, and all were randomised clinical trials. Twenty‐four studies were double‐masked, and three were single‐masked; 17 were placebo‐controlled, and the mean follow‐up was 59.5 (SD 74.2) days. The mean wash‐out from previous therapies was 18.3 (36.9) days. A total of 1092 patients and 2184 eyes were enrolled in the studies. The mean age was 13.3 (4.5) years old. The severity of disease was considered in only 13 of the 27 studies, and only five trials reported in which period of the year the study was conducted. Four studies were sponsored by a pharmaceutical company.
Nineteen of these 27 studies, of which two were with three groups of participants, compared mast cell stabilisers to another drug: ten groups versus placebo; eight versus another mast cell stabiliser; two versus corticosteroids; one versus an anithistaminic. Five studies compared an immunomodulator to another drug: four versus placebo and one versus a mast cell stabiliser. Two studies, of which one with three groups of participants, compared a NSAID to another drug: two versus placebo and one versus corticosteroids. One study compared an antimitotic drug to placebo (table 1).
Table 1 Characteristics and findings of randomised clinical trials included in the systematic review of 27 studies.
| Study | Drugs | Treatment (drops/day; duration) | Efficacy in reducing signs and symptoms (significant difference) |
|---|---|---|---|
| Akpek et al31 | Mitomycin‐C 0,01% (G1) vs placebo (G2) | 3; 14 days | G1>G2: photophobia, tarsal papillae, corneal involvement, limbal disease, hyperaemia |
| Gunduz et al26 | Lodoxamide 0,1% (G1) vs NAAGA (G2) | 4; 60 days | G1>G2: itching, tearing, photophobia, tarsal papillae, corneal involvement |
| Caldwell et al20 | Lodoxamide 0,1% (G1) vs cromolyn sodium 4% (G2) | 4; 28 days | G1>G2: itching, tearing, tarsal papillae, limbal disease, hyperaemia |
| Bonini et al19 | Nedocromil sodium 2% (G1) vs placebo (G2) | 4; 42 days | G1>G2: itching, tearing, hyperaemia |
| Leonardi et al27 | Lodoxamide 0,1% (G1) vs sodium cromoglycate 4% | 4; 10 days | G1>G2: total signs and symptoms |
| Foster and Duncan12 | Cromolyn sodium 4% (G1) vs placebo (G2) | 4; 42 days | G1>G2: itching, corneal involvement, hyperaemia |
| Pucci et al34 | Ciclosporin 2% (G1) vs placebo (G2) | 4; 14 days | G1>G2: total signs and symptoms |
| Foster16 | Cromolyn sodium 4% (G1) vs placebo (G2) | 4; 42 days | G1>G2: itching, tearing, tarsal papillae, corneal involvement, limbal disease, hyperaemia |
| Dahan and Appel15 | Sodium cromoglycate (G1) vs dexamethasone 0,1% (G2) vs placebo (G3) | 4; 28 days | G1>G3; G2 vs G3; G1+G2 vs G3: total signs |
| El Hennawi22 | Sodium cromoglycate 2% (G1) vs nedocromil sodium 2% (G2) vs placebo (G3) | 4; 28 days | G1>G3; G2 > G3: total signs and symptoms, limbal disease |
| Verin et al33 | Lodoxamide 0,1% (G1) vs Levocabastine 0,05% (G2) | 4; 90 days | G1>G2: total signs and symptoms, itching, tearing, photophobia, tarsal papillae, hyperaemia |
| Santos et al23 | Lodoxamide 0,1% (G1) vs placebo (G2) | 4; 90 days | G1>G2: total signs and symptoms, tarsal papillae, corneal involvement, limbal disease |
| Tabbara and Arafat11 | Cromolyn sodium 2% (G1) vs placebo (G2) | 4; 365 days | G1>G2: total symptoms, corneal involvement, limbal disease |
| Bleik and Tabbara18 | Ciclosporin 2% (G1) vs placebo (G2) | 4; 42 days | G1>G2: itching, photophobia, corneal involvement, limbal disease, hyperaemia |
| Fahy et al21 | Lodoxamide 0,1% (G1) vs cromoglycate 2% (G2) | 4; 28 days | G1>G2: total signs and symptoms |
| Secchi et al17 | Ciclosporin 2% (G1) vs placebo (G2) | 4; 15 days | G1>G2: total signs and symptoms |
| Tabbara and Al‐Kharashi29 | Nedocromil sodium 2% (G1) vs fluorometholone 0,1% (G2) | 4; 14 days | G2>G1: limbal disease, ocular surface temperature |
| Verin et al30 | Nedocromil sodium 2% (G1) vs sodium cromoglicate 2% (G2) | 4; 154 days | G1>G2: photophobia, tarsal papillae, corneal involvement, hyperaemia |
| Centofanti et al25 | Mipragoside 0,5% (G1) vs placebo (G2) | 4; 14 days | G1>G2: total signs and symptoms, itching, hyperaemia |
| Gupta et al32 | Ciclosporin 2% (G1) vs placebo (G2) | 4; 120 days | G1>G2: total signs |
| Easty et al10 | Disodium cromoglycate 1% (G1) vs placebo (G2) | 4; 42 days | G1>G2: total signs and symptoms |
| El Hennawi14 | Disodium cromoglycate 2% (G1) vs 4% (G2) | 4; 42 days | No statistically significant differences reported |
| Kosrirukvongs et al35 | Ciclosporin 0,5% (G1) vs lodoxamide (G2) | 4; 30 days | No statistically significant differences reported |
| Sud et al24 | Flurbiprofen 0,03% (G1) vs bethametasone 0,1% (G2) vs placebo (G3) | 4; 42 days | G1+G2>G3: total signs and symptoms |
| Sharma et al28 | Ketorolac 0,5% (G1) vs placebo (G2) | 4; 14 days | G1>G2: itching |
| Baryishak et al13 | Sodium cromoglycate 2% (G1) vs placebo (G2) | 4; 14 days | G1>G2: total signs |
| Avunduk9 | Lodoxamide 0,1% (G1) vs cromolyn sodium 4% (G2) | 8; 10 days | G1>G2: total signs and symptoms |
Out of these 27 potentially relevant randomised clinical trials, only 10 were included in the meta‐analysis because they were suitable for statistical analysis (table 2).10,11,12,16,17,18,25,28,31,34.
Table 2 Baseline characteristics of the randomised clinical trials evaluating the efficacy of topical treatment for vernal keratoconjunctivitis.
| Trial | Country | Intervention | No of patients at baseline | Final no of patients | Outcome measure | Quality score* |
|---|---|---|---|---|---|---|
| Easty et al10 | UK | Sodium cromoglycate 1% vs placebo | 22 | 22 | Signs, symptoms | 9 |
| Tabbara and Arafat11 | Lebanon | Sodium cromoglycate 2% vs placebo | 14 | 14 | Signs | 9 |
| Foster and Duncan12 | USA | Sodium cromoglycate 4% vs placebo | 11 | 11 | Signs, symptoms | 9 |
| Foster16 | USA | Sodium cromoglycate 4% vs placebo | 72 | 65 | Signs, symptoms | 9 |
| Secchi et al17 | Italy | Ciclosporin 2% vs placebo | 11 | 9 | Signs, symptoms | 9 |
| Bleik and Tabbara18 | Saudi Arabia | Ciclosporin 2% vs placebo | 20 | 20 | Signs, symptoms | 9 |
| Centofanti et al25 | Italy | Mipragoside 0.5% vs placebo | 24 | 20 | Signs, symptoms | 9 |
| Sharma et al28 | India | Ketorolac 0.5% vs placebo | 21 | 21 | Signs, symptoms | 9 |
| Akpek et al31 | Turkey | Mitomycin‐C 0.01% vs placebo | 26 | 26 | Signs, symptoms | 8 |
| Pucci et al34 | Italy | Ciclosporin 2% vs placebo | 24 | 24 | Signs, symptoms | 9 |
*Delphi List (0–9).
Two hundred and forty‐five patients were enrolled in these 10 trials. The mean age was 13.3 (2.8) years. The design and general characteristics of these studies are listed in table 3.
Table 3 Design and general characteristics of selected randomised clinical trials evaluating the efficacy of topical treatment for vernal keratoconjunctivitis: number and per cent of RCTs reporting the design information.
| No. | Per cent | |
|---|---|---|
| RCTs | 10 | 100 |
| Inclusion criteria reported | 10 | 100 |
| Diagnostic criteria | ||
| History | 4 | 40 |
| Signs/symptoms | 8 | 80 |
| Laboratory | 1 | 10 |
| Drug class | ||
| Mast cell stabilisers | 5 | 50 |
| Immunomodulators | 3 | 30 |
| NSAIDs | 1 | 10 |
| Antimitotic | 1 | 10 |
| Duration of treatment | 10* | 100 |
| Wash‐out | 8† | 80 |
| Follow‐up: | 10‡ | 100 |
| Type of outcome assessed | ||
| Symptoms | 0 | 0 |
| Signs | 1 | 10 |
| Signs and symptoms | 9 | 90 |
| Severity | 4 | 40 |
| Sample size: | ||
| Participants | 245 | – |
| Eyes | 490 | – |
*Duration of treatment: 60 (108) days (mean (SD)); †wash‐out: 13 (7.6) days (mean (SD)); ‡follow‐up: 62 (107) days (mean (SD)).
NSAIDs, non‐steroidal anti‐inflammatory drugs; RCTs, randomised clinical trials
There was no significant difference in the quality of the studies as assessed by the Delphi List, and so the weight of each single study was primarily based on the number of participants.
Outcomes reported and evaluated in our meta‐analysis included a total score for signs and symptoms and single scores for the following specific signs and symptoms: itching, tearing, photophobia, hyperaemia, tarsal papillae, limbal disease, and corneal involvement.
Efficacy of topical treatment for vernal keratoconjunctivitis
The effect of topical mast cell stabilisers, immunomodulators, NSAIDs and antimitotic agents in controlling signs and symptoms of patients with VKC, compared with placebo, was evaluated in the trials included in both the systematic review and the meta‐analysis.
Systematic review of the initial 27 studies indicated a positive effect of common antiallergic eye‐drops in reducing signs and symptoms of the disease (table 1).
All the drugs tested in these 27 studies were found to be safe, well tolerated (except only transient mild burning and tearing upon instillation of ciclosporin eye‐drops) and more effective than placebo. A greater number of studies (20) evaluated the efficacy of common antiallergic eye‐drops (levocabastine, lodoxamide, mipragoside, NAAGA, nedocromil sodium, sodium/disodium cromoglycate). Among these, lodoxamide appeared to be the most effective. Compared with antiallergic drugs, however, corticosteroids (one study) were more effective, while immunomodulators (1 study) did not show a statistically significant difference in reduction of signs and symptoms of the disease.
To demonstrate whether the efficacy of these drugs in reducing signs and symptoms of active disease was significant, a meta‐analysis was conducted on the 10 trials that presented comparable data suitable for statistical analysis.
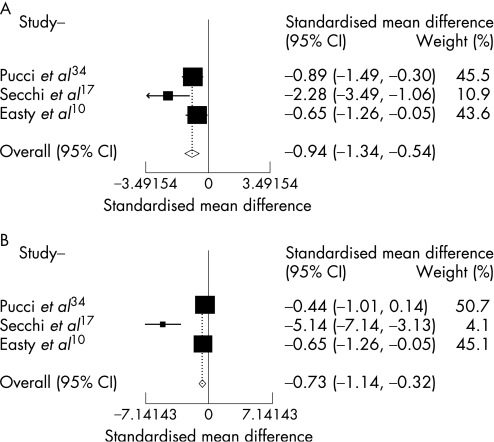
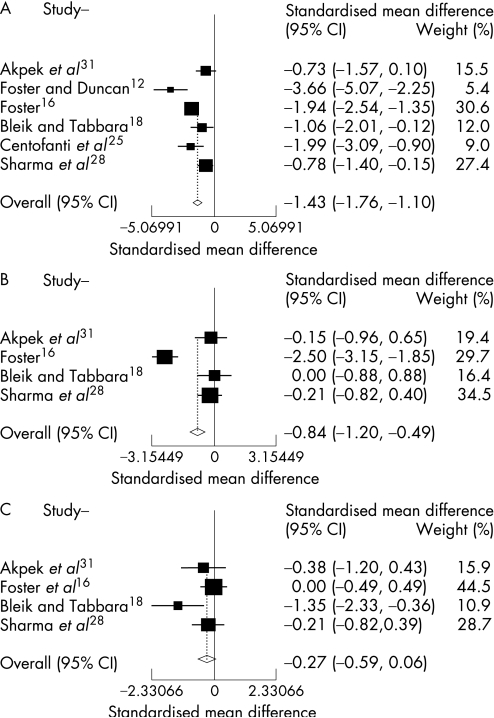
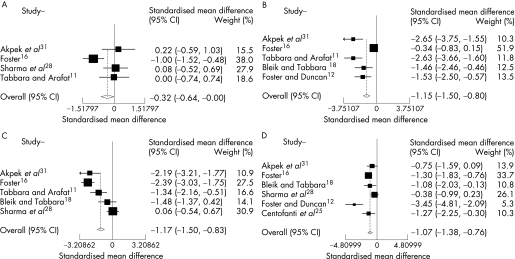
The combined results of these 10 RCTs included in the meta‐analysis clearly demonstrated a positive effect favouring improvement of signs and symptoms in active VKC after topical therapy, independent of the pharmaceutical class for the following clinical variables: total signs and symptoms, itching, tearing, corneal involvement, limbal disease and hyperaemia. A positive effect in reducing tarsal papillae was also demonstrated, but it was only slightly significant (p = .047). There was a trend towards improvement also in photophobia, but it was not statistically significant (p = .105) (table 4 and figs 2–4).
Table 4 Combined results from randomised clinical trials examining the absolute change in specific signs and symptoms after topical treatment versus placebo.
| Clinical variable | No of studies | SMD | 95% CI | p Value | Publications |
|---|---|---|---|---|---|
| Total signs | 3 | −0.94 | −1.34 to −0.54 | <0.001 | 10,17,34 |
| Total symptoms | 3 | −0.73 | −1.14 to −0.32 | <0.001 | 10,17,34 |
| Itching | 6 | −1.43 | −1.76 to −1.1 | 0.001 | 12,16,18,25,28,31 |
| Tearing | 4 | −0.84 | −1.2 to −0.49 | <0.001 | 16,18,28,31 |
| Photophobia | 4 | −0.27 | −0.59 to 0.06 | 0.105 | 16,18,28,31 |
| Tarsal papillae | 4 | −0.32 | −0.64 to 0 | 0.047 | 11,16,28,31 |
| Corneal involvement | 5 | −1.15 | −1.5 to −0.8 | <0.001 | 11,12,16,18,31 |
| Limbal disease | 5 | −1.17 | −1.50 to −0.83 | <0.001 | 11,16,18,28,31 |
| Hyperaemia | 6 | −1.07 | −1.38 to −0.76 | <0.001 | 12,16,18,25,28,31 |
SMD, standardised mean difference.
Figure 2 Combined results from randomised clinical trials evaluating the efficacy of topical therapy for vernal keratoconjunctivitis versus placebo on total signs (A): significant efficacy (p<0.001), not significant heterogeneity (p = 0.98) and total symptoms (B): significant efficacy (p<0.001), not significant heterogeneity (p = 0.99).
Figure 3 Combined results from randomised clinical trials evaluating the efficacy of topical therapy for vernal keratoconjunctivitis versus placebo on individual symptoms. Itching (A): significant efficacy (p = 0.001), not significant heterogeneity (p>0.99); tearing (B): significant efficacy (p<0.001), not significant heterogeneity (p>0.99); photophobia (C): not significant efficacy (p = 0.105), not significant heterogeneity (p = 0.99).
Figure 4 Combined results from randomised clinical trials evaluating the efficacy of topical therapy for vernal keratoconjunctivitis versus placebo on individual signs. Tarsal papillae (A): significant efficacy (p = 0.047), not significant heterogeneity (p>0.99); corneal involvement (B): significant efficacy (p<0.001), not significant heterogeneity (p>0.99); limbal disease (C): significant efficacy (p<0.001), not significant heterogeneity (p = 0.99); hyperaemia (D): significant efficacy (p<0.001), not significant heterogeneity (p>0.99).
Comparison among different pharmaceutical classes and drugs was not possible due to the small number of available RCTs on the subject and the great variability in assessment of outcome measures to evaluate the effects of topical treatment for VKC
Discussion
A systematic review of randomised clinical trials aimed at evaluating topical therapies for the treatment of VKC indicated that all the common antiallergic eye‐drops are effective in reducing the signs and symptoms of the disease. Topical antiallergic agents used by VKC patients are the same as those used to treat other forms of allergic conjunctivitis, since the exact pathogenesis of VKC is unknown, and there is, thus, no specific treatment.3 Although these topical drugs do not completely control the disease, they have been shown to be effective in reducing signs and symptoms during active phases by interfering with at least one pathogenic mechanism.5,36 Even placebo is known to have beneficial effects, improving signs and symptoms by acting as a lubricant of the ocular surface. Nevertheless, several reports indicate that some patients do not respond to antiallergic treatment and respond only to topical steroids: in fact, particularly moderate to severe cases may not respond to common antiallergic treatments and may also require new therapeutic strategies.
These observations underlined the need for a meta‐analysis to evaluate the efficacy of all the currently available topical treatments for VKC: to undertake this meta‐analysis, we selected only clinical trials conducted on patients diagnosed as having VKC, and we used strict eligibility criteria to evaluate the combined results of comparable RCTs only. We found that a very limited number of studies specifically focused on VKC, and even fewer were considered suitable. Clinical trials of antiallergic agents for the treatment of other types of ocular allergies were excluded.
Despite the considerable available literature providing possible protocol standards for the assessment of drug efficacy,37,38 the majority of studies on VKC were not randomised and presented inadequate control groups, and most were not multicentred. As a result, small sample sizes made it difficult to detect small to moderate, but potentially clinically relevant, differences between the treatments tested. These limitations also made it impossible to compare different pharmaceutical classes and/or individual drugs used for the treatment of this disease.
Heterogeneity among the trials' findings lent uncertainty as to whether it was appropriate to pool and summarise the selected studies. However, heterogeneity was tested in this meta‐analysis, and was found to not be statistically significant, indicating that pooling of the studies' results was methodologically correct.39,40 Overall, this meta‐analysis of RCTs demonstrates that currently available topical therapies (mast cell stabilisers, immunomodulators, NSAIDs and antimitotic) are significantly more effective than placebo for treating most signs and symptoms of vernal keratoconjunctivitis. However, there is a lack of evidence supporting the recommendation of one specific type of medication for treating this disorder.
These findings indicate that, to study a homogeneous group of patients, general consensus on the clinical stages of VKC should be defined. This disease is of widely varying severity due to differences in individual sensitivity, geographical localisation, time of observation, and many environmental factors that greatly influence the outcome of clinical trials. We suggest that more detailed diagnostic criteria be added to protocols to better define and exclude other allergic entities and to better define the clinical stages of VKC.
Abbreviations
NSAID - non‐steroidal anti‐inflammatory drug
RCT - randomised clinical trial
VKC - vernal keratoconjunctivitis
Footnotes
Financial support: None.
Competing interests: None.
References
- 1.Bonini S, Bonini S, Lambiase A.et al Vernal keratoconjunctivitis revisited: a case series of 195 patients with long‐term follow‐up. Ophthalmology 20001071157–1163. [DOI] [PubMed] [Google Scholar]
- 2.Leonardi A, Secchi A G. Vernal keratoconjunctivitis. Int Ophthalmol Clin 20034341–58. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res 200221319–339. [DOI] [PubMed] [Google Scholar]
- 4.Allansmith M R, Ross R N. Ocular allergy and mast cell stabilizers. Surv Ophthalmol 198630229–244. [DOI] [PubMed] [Google Scholar]
- 5.Bielory L. Allergic diseases of the eye. Med Clin North Am 200690129–148. [DOI] [PubMed] [Google Scholar]
- 6.Green S, Higgins J.Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 , Section 7.5. http://www.cochrane.org/resources/handbook/ (accessed 1 Oct 2007)
- 7.Verhagen A P, Vet H C W, Bie R A.et al The Delphi List: a criteria list for quality assessment of randomized clinical trial for conducting systematic reviews developed by Delphi Consensus. J Clin Epidemiol 1998511235–1241. [DOI] [PubMed] [Google Scholar]
- 8. STATA [computer program] Release 8.0. College Station: Stata 2003
- 9.Avunduk A M, Avunduk M C, Kapicioglu Z.et al Mechanisms and comparison of anti‐allergic efficacy of topical lodoxamide and cromolyn sodium treatment in vernal keratoconjunctivitis. Ophthalmology 20001071333–1337. [DOI] [PubMed] [Google Scholar]
- 10.Easty D L, Rice N S, Jones B R. Clinical trial of topical disodium cromoglycate in vernal kerato‐conjunctivitis. Clin Allergy 1972299–107. [DOI] [PubMed] [Google Scholar]
- 11.Tabbara K F, Arafat N T. Cromolyn effects on vernal keratoconjunctivitis in children. Arch Ophthalmol 1977952184–2186. [DOI] [PubMed] [Google Scholar]
- 12.Foster C S, Duncan J. Randomized clinical trial of topically administered cromolyn sodium for vernal keratoconjunctivitis. Am J Ophthalmol 198090175–181. [DOI] [PubMed] [Google Scholar]
- 13.Baryishak Y R, Zavaro A, Monselise M.et al Vernal keratoconjunctivitis in an Israeli group of patients and its treatment with sodium cromoglycate. Br J Ophthalmol 198266118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Hennawi M. A comparison between 2% and 4% sodium cromoglycate eye drops in the treatment of vernal keratoconjunctivitis. Curr Eye Res. 1982/ 19832765–768. [DOI] [PubMed] [Google Scholar]
- 15.Dahan E, Appel R. Vernal keratoconjunctivitis in the black child and its response to therapy. Br J Ophthalmol 198367688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster C S. Evaluation of topical cromolyn sodium in the treatment of vernal keratoconjunctivitis. Ophthalmology 198895194–201. [DOI] [PubMed] [Google Scholar]
- 17.Secchi A G, Tognon M S, Leonardi A. Topical use of cyclosporine in the treatment of vernal keratoconjunctivitis. Am J Ophthalmol 1990110641–645. [DOI] [PubMed] [Google Scholar]
- 18.Bleik J H, Tabbara K F. Topical cyclosporine in vernal keratoconjunctivitis. Ophthalmology 1991981679–1684. [DOI] [PubMed] [Google Scholar]
- 19.Bonini S, Barney N P, Schiavone M.et al Effectiveness of neodocromil sodium 2% eyedrops on clinical symptoms and tear fluid cytology of patients with vernal conjunctivitis. Eye 19926648–652. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell D R, Verin P, Hartwich‐Young R.et al Efficacy and safety of lodoxamide 0.1% vs cromolyn sodium 4% in patients with vernal keratoconjunctivitis. Am J Ophthalmol 1992113632–637. [DOI] [PubMed] [Google Scholar]
- 21.Fahy G T, Easty D L, Collum L M T.et al Randomised double‐masked trial of lodoxamide and sodium cromoglycate in allergic eye disease. A multicentre study. Eur J Ophthalmol 19922144–149. [DOI] [PubMed] [Google Scholar]
- 22.El Hennawi M. A double blind placebo controlled group comparative study of ophthalmic sodium cromoglycate and nedocromil sodium in the treatment of vernal keratoconjunctivitis. Br J Ophthalmol 199478365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos C I, Huang A J, Abelson M B.et al Efficacy of lodoxamide 0.1% ophthalmic solution in resolving corneal epitheliopathy associated with vernal keratoconjunctivitis. Am J Ophthalmol 1994117488–497. [DOI] [PubMed] [Google Scholar]
- 24.Sud R N, Greval R S, Bajwa R S. Topical flurbiprofen therapy in vernal keratoconjunctivitis. Indian J Med Sci 199549205–209. [PubMed] [Google Scholar]
- 25.Centofanti M, Schiavone M, Lambiase A.et al Efficacy of mipragoside ophthalmic gel in vernal keratoconjunctivitis. Eye 199610422–424. [DOI] [PubMed] [Google Scholar]
- 26.Gunduz K, Ucakhan O, Budak K.et al Efficacy of lodoxamide 0.1% versus N‐acetyl aspartyl glutamic acid 6% ophthalmic solutions in patients with vernal keratoconjunctivitis. Ophthalmic Res 19962880–87. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi A, Borghesan F, Avarello A.et al Effect of lodoxamide and disodium cromoglycate on tear eosinophil cationic protein in vernal keratoconjunctivitis. Br J Ophthalmol 19978123–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A, Gupta R, Ram J.et al Topical ketorolac 0.5% solution for the treatment of vernal keratoconjunctivitis. Indian J Ophthalmol 199745177–180. [PubMed] [Google Scholar]
- 29.Tabbara K F, Al‐Kharashi S A. Efficacy of nedocromil 2% versus fluorometholone 0.1%: a randomised, double masked trial comparing the effects on severe vernal keratoconjunctivitis. Br J Ophthalmol 199983180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verin P H, Dicker I D, Mortemousque B. Nedocromil sodium eye drops are more effective than sodium cromoglycate eye drops for the long‐term management of vernal keratoconjunctivitis. Clin Exp Allergy 199929529–536. [DOI] [PubMed] [Google Scholar]
- 31.Akpek E K, Hasiripi H, Christen W G.et al A randomized trial of low‐dose, topical mitomycin‐C in the treatment of severe vernal keratoconjunctivitis. Ophthalmology 2000107263–269. [DOI] [PubMed] [Google Scholar]
- 32.Gupta V, Sahu P K. Topical cyclosporin A in the management of vernal keratoconjunctivitis. Eye 20011539–41. [DOI] [PubMed] [Google Scholar]
- 33.Verin P, Allewaert R, Joyaux J C.et al Comparison of lodoxamide 0.1% ophthalmic solution and levocabastine 0.05% ophthalmic suspension in vernal keratoconjunctivitis. Eur J Ophthalmol 200111120–125. [DOI] [PubMed] [Google Scholar]
- 34.Pucci N, Novembre E, Cianferoni A.et al Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol 200289298–303. [DOI] [PubMed] [Google Scholar]
- 35.Kosrirukvongs P, Vichyanond P, Wongsawad W. Vernal keratoconjunctivitis in Thailand. Asian Pac J Allergy Immunol 20032125–30. [PubMed] [Google Scholar]
- 36.Owen C G, Shah A, Henshaw K.et al Topical treatments for seasonal allergic conjunctivitis: systematic review and meta‐analysis of efficacy and effectiveness. Br J Gen Pract 200454412–414. [PMC free article] [PubMed] [Google Scholar]
- 37.Egger M, Smith G D, Phillips A N. Meta‐analysis: principles and procedures. BMJ 19973151533–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger V W, Christophi C A. Randomization technique, allocation concealment, masking, and susceptibility of trials to selection bias. J Mod Appl Stat Meth 2003280–86. [Google Scholar]
- 39.Thompson S G. While sources of heterogeneity in meta‐analysis should be investigated. BMJ 19943091351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton A J, Abrams K R, Jones D R.et alMethods for meta‐analysis in medical research. New York: Wiley, 2000