Abstract
Aim
To evaluate central retinal thickness and foveal function using optical coherence tomography (OCT) and the Rarebit Fovea Test (RFT) in patients with diabetes without previously known retinopathy or maculopathy.
Method
Forty‐two patients with diabetes mellitus (DM) were selected from the screening records at St Erik Eye Hospital. Inclusion criteria were absence of macular or other retinal changes at previous screening examination and best corrected visual acuity ⩾1.0. These patients and 42 healthy controls were examined with the recently developed RFT, and retinal thickness was measured using OCT. Lens thickness and light scatter were evaluated by Scheimpflug photography.
Results
Significantly more DM subjects (12/42) had a subnormal RFT result compared with the controls (2/42) (p = 0.007). None of the 12 DM subjects had maculopathy, one had mild non proliferative diabetic retinopathy, and five had minimal non‐proliferative diabetic retinopathy. The retinal thickness in the pericentral zone was significantly (p<0.05) thinner in DM patients with subnormal RFT compared with the controls.
Conclusion
Decreased RT and subnormal RFT results were found in a subgroup of diabetes patients, despite normal screening results. Prospective studies are under way to evaluate the prognostic implications.
Standard procedures for diabetes mellitus (DM) screening are fundus photography or funduscopy with dilated pupils. However, there is evidence suggesting that neuronal changes have an important role in the development of diabetic retinopathy (DRP) and that retinal degeneration may precede visible or vascular changes.1,2 In a recent study, the retinal thickness was found to be decreased in subjects with DM type 1 and minimal DRP compared with healthy controls.3
The Rarebit technique including perimetry and the Rarebit Fovea Test (RFT) was recently developed with the explicit aim to reveal low degree damage of the visual system.4 In a previous study,5 the effect of RFT stimulus luminance on examination results was studied, and the limits for normality established. The aim of the current study was to evaluate the ability of RFT to detect foveal dysfunction in DM patients without previously known retinopathy or maculopathy.
Materials and methods
Forty‐two consecutive patients with DM were recruited from the Retina Clinic, St Erik Eye Hospital, Stockholm, Sweden. Inclusion criteria were no previous known macular or other retinal changes, best corrected Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity (BCVA) of ⩾1.0, refractive error within ±6 dioptres and no ophthalmic or systemic disease other than DM. Additionally, 42 healthy, gender‐ and age‐matched individuals, fulfilling the same inclusion criteria, served as controls. Clinical data are given in table 1.
Table 1 Data from the two examined groups.
| DM | Control | p | |
|---|---|---|---|
| Number | 42 | 42 | |
| Gender females/males | 15/27 | 24/18 | 0.08 |
| Mean age (years) (SD) | 40.5 (9.6) | 40.0 (10.4) | 0.82 |
| Median ETDRS VA (range) | 1.2 (1.0 to 2.0) | 1.6 (1.0 to 2.0) | 0.08 |
| DM type 1/DM type 2 | 27/15 | – | – |
| Median DM duration (years) (range) | 10 (0 to 29) | – | – |
DM, diabetes mellitus; VA, ETDRS visual acuity.
All subjects underwent an ophthalmic examination, including RFT, fundus and lens photography and OCT examination.
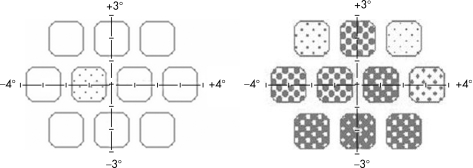
The RFT test is included in the Rarebit Perimetry program package4 (see http://www.oft.gu.se/webdiagnos/) and runs on a personal computer with a liquid crystal display. The test principle is to briefly (200 ms) present zero, one or two bright and very small (<0.5 min of arc) dots against a dark background in a completely dark room. The test task is to fixate a small, flickering cross in the middle of the screen and to respond by one or two mouse clicks when one or two test stimuli are detected anywhere on the screen. The test time is approximately 90 s, and the result is presented as mean hit rate (MHR), that is the number of stimuli seen relative to stimuli presented (fig 1). Based on data from a previous study,5 the stimulus luminance was set to 68 cd/m2, expected to give a MHR close to 100%, that is 97% or better, in normal subjects.
Figure 1 RFT results from one control and one diabetic subject. Empty squares indicate that all dots have been perceived within the test area. The fraction of missed targets is proportionally depicted by a grey scale in the test area. In the example from a normal subject to the left, only one dot was missed in one area, and the mean hit rate was 99%. In the example to the right, from a diabetic subject, the mean hit rate was 54%.
High‐resolution fundus photographs were obtained with dilated pupils, using a Zeiss FF450plus IRu™ (Jena DE) fundus camera. The Visupac 3.5 Software™ (Pirmasens, DE) was used for analysing the images. The severity of DRP and diabetic maculopathy was classified by one of the authors (GvW), based on the criteria of the ETDRS protocol.6
Lens thickness and light scatter were evaluated by Scheimpflug photography, using the Nidek, EAS‐1000™ (Nidek Inc), after pupil dilation. The distance from the anterior lens capsule to the central clear zone (zone A–E) was used as a measure of lens thickness.7 The lens light scatter was measured in the anterior adult nuclear area, including a part of the deep cortical layer, since it is assumed that this layer should have the largest influence on the overall optical quality of the lens.7
The macular thickness was measured with optical coherence tomography using the Stratus OCT™, model 3000 (Carl Zeiss Meditec Int.) with a dilated pupil. Six radial OCT scans were obtained in the centre of the macula. For analysis of the RT, the macula was divided into three areas: the fovea with a diameter of 1 mm, the pericentral area (doughnut‐shaped ring with an inner diameter of 1 mm and an outer of 3 mm) and the peripheral area (inner diameter of 3 mm and an outer of 6 mm). Both eyes in all subjects were examined in random order, and the results from right eyes were used for analysis.
The study was approved by the local ethical committee and performed according to the Helsinki declaration. Written informed consent was obtained from all participants.
For statistical analysis, the Student t test, a one‐way ANOVA test and the Fisher test were used. A p value of <0.05 was regarded as significant.
Results
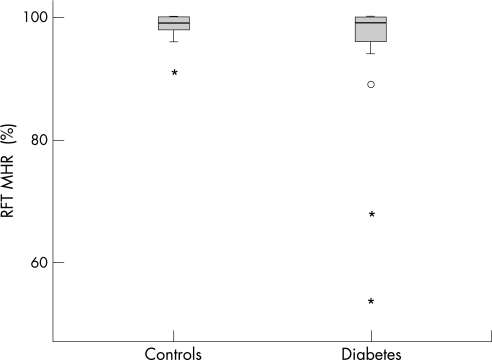
There was a significant (p = 0.03) difference between the groups regarding MHR; the mean MHR for the DM group was 96 (9)% and for controls 99 (2)%. Also, significantly more subjects with DM (12/42) had a subnormal RFT (<97%) compared with the controls (2/42) (p = 0.007; see fig 2). None of the 12 DM subjects with abnormal RFT had maculopathy, one had mild non‐proliferative diabetic retinopathy, and five had minimal non‐proliferative diabetic retinopathy. The retinal thickness measured in the pericentral zone was significantly (p<0.05) thinner in DM subjects with subnormal MHR compared with controls (see table 2), but there was no significant correlation between RFT findings and OCT measurements.
Figure 2 Box plot of Rarebit Fovea Test (RFT) findings from the examined groups. MHR, mean hit rate; %, percentage of stimuli seen relative to stimuli presented. Box, interquartile distances; bold line inside box, median; °, outlier; *, extreme value.
Table 2 Retinal thickness.
| OCT zone | DM subnormal RFT (n = 12) | DM normal RFT (n = 30) | Control (n = 42) |
|---|---|---|---|
| Fovea | 212.25 (17.96) | 212.87 (16.69) | 212.40 (19.35) |
| Pericentral | 272.90* (13.59) | 282.42 (12.68) | 283.88* (13.34) |
| Peripheral | 234.54 (14.11) | 239.15 (16.59) | 241.53 (11.54) |
*Statistically significant difference (one‐way ANOVA) (p<0.05).
Retinal thickness (µm), mean (SD). Fovea, fovea zone with a diameter of 1 mm; Pericentral, pericentral zone with a diameter of 1–3 mm; Peripheral, peripheral zone with a diameter of 3–6 mm.
One of the two controls with subnormal RFT (MHR = 91%) had drusen. In the other subject (MHR = 96%) no ocular abnormalities were detected. Among the subjects with normal RFT results, 14 of the 42 controls had drusen or age‐related pigment epithelial defects, and one subject with DM was classified as having a mild maculopathy and four had drusen. None of the studied parameters showed any correlation with age, duration or type of DM.
Analysis of the Scheimpflug images revealed no significant difference between the DM group and the control group regarding lens light scatter and thickness, and these data are not further reported here.
Discussion
The current study reports reduced RFT mean hit rate in a subgroup of patients with DM with no or mild retinopathy. This observation supports the RFT concept, that is detection of low‐degree neural damage.4 The test principle, with very small stimuli, is assumed to test the integrity of the retino‐cortical detector matrix, in the fovea defined by photoreceptor density. The signal from these receptors is relayed through the Henle loops to the perifoveal ganglion cells, stacked on top of each other.8 The demonstration by OCT that the pericentral retinal area is significantly thinner in the DM patients is in agreement with findings by Biallosterski and co‐workers,3 and confirms the relevance of the RFT results. These signs of neural degeneration can be present without visible vascular changes, since the fundus photographs did not show any abnormalities in 6 of the 12 subjects with subnormal RFT results. This observation is in line with recent findings in studies of retinal changes in DM using histology, electrophysiology and nerve‐fibre layer imaging.2,9,10 The reverse finding, normal RFT and mild maculopathy was observed in only one DM subject. In none of the patients with diabetes was fluorescein angiography considered to be indicated. Fundus photography revealed non‐diabetic abnormalities in almost 30% of healthy controls. Hard drusen are common in subject above 40 years of age11 and are, as an isolated finding, not regarded as a sign of macular degeneration.12
The RFT is designed to detect small defects in the retino‐cortical detector layer, which is assumed to normally be complete. To achieve this, the test target should, in theory, stimulate only one receptive field. Owing to the very high cone density in the fovea, this is a technical challenge.13,14 Makous and co‐workers15 recently used adaptive optics and microflashes in a study of a subject with genetically determined absence of green‐sensitive cones. With a small bright stimulus, subtending 0.75′ and with the use of adaptive optics to minimise the influence of the aberrations of the eye's optical system, they managed to detect scotomas as small as the size of one photoreceptor. The results of this and the present study indicate that psychophysical techniques, using very small stimuli, may be a useful method for the detection of very small defects in the fovea.
Conclusion
Macular changes, not detected by screening methods, could be demonstrated using both structural and functional tests in a subgroup of patients with DM with no or minimal retinopathy. Prospective studies are under way to evaluate the prognostic implications of these observations.
Abbreviations
BCVA - best corrected Early Treatment Diabetic Retinopathy Study visual acuity
DM - diabetes mellitus
DRP - diabetic retinopathy
ETDRS - Early Treatment Diabetic Retinopathy Study
MHR - mean hit rate
OCT - optical coherence tomography
RFT - Rarebit Fovea Test
Footnotes
Competing interests: None declared.
References
- 1.Lieth E, Gardner T W, Barber A J.et al (The Penn State Retina Research Group). Retinal neurodegeneration: early pathology in diabetes. Clin Exp Ophthalmol 2000283–8. [DOI] [PubMed] [Google Scholar]
- 2.Barber A J. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 200327283–290. [DOI] [PubMed] [Google Scholar]
- 3.Biallosterski C, van Velthoven M E, Michels R P.et al Decreased OCT‐measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol 2007911135–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisen L. New, sensitive window on abnormal spatial vision: rarebit probing. Vision Res 2002421931–1939. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson M, Wanger P, Martin L. Perception of very small visual stimuli in the fovea: normative data for the Rarebit Foveal Test. Clin Exp Optom 20068981–85. [DOI] [PubMed] [Google Scholar]
- 6.von Wendt G, Heikkila K, Summanen P. Assessment of diabetic retinopathy using two‐field 60 degrees fundus photography. A comparison between red‐free, black‐and‐white prints and colour transparencies. Acta Ophthalmol Scand 199977638–647. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki H, Hockwin O, Kasuga T.et al An index for human lens transparency related to age and lens layer: Comparison between normal volunteers and diabetic patients with still clear lenses. Ophthalmic Res 19993193–103. [DOI] [PubMed] [Google Scholar]
- 8.Drasdo N, Millican C L, Katholi C R.et al The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res 2007472901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes de Faria J M, Russ H, Costa V P. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol 200286725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parisi V, Uccioli L. Visual electrophysiological responses in persons with type 1 diabetes [Review]. Diabetes Metab Res Rev 20011712–18. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Davis D, Magli Y L.et al The Wisconsin age‐related maculopathy grading system. Ophthalmology 1991981128–1134. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein B E, Jensen S C.et al The five‐year incidence and progression of age‐related maculopathy: the Beaver Dam Eye Study. Ophthalmology 19971047–21. [DOI] [PubMed] [Google Scholar]
- 13.Sjöstrand J, Olsson V, Popovic Z.et al Quantitative estimations of foveal and extra‐foveal retinal circuitry in humans. Vision Res 1999392987–2998. [DOI] [PubMed] [Google Scholar]
- 14.Curcio A C, Allen A K. Topography of ganglion cells in human retina. J Comp Neurol 19903005–25. [DOI] [PubMed] [Google Scholar]
- 15.Makous W, Carroll J, Wolfing J I.et al Retinal microscotomas revealed with adaptive‐optics microflashes. Invest Ophthalmol Vis Sci 2006474160–4167. [DOI] [PubMed] [Google Scholar]