Abstract
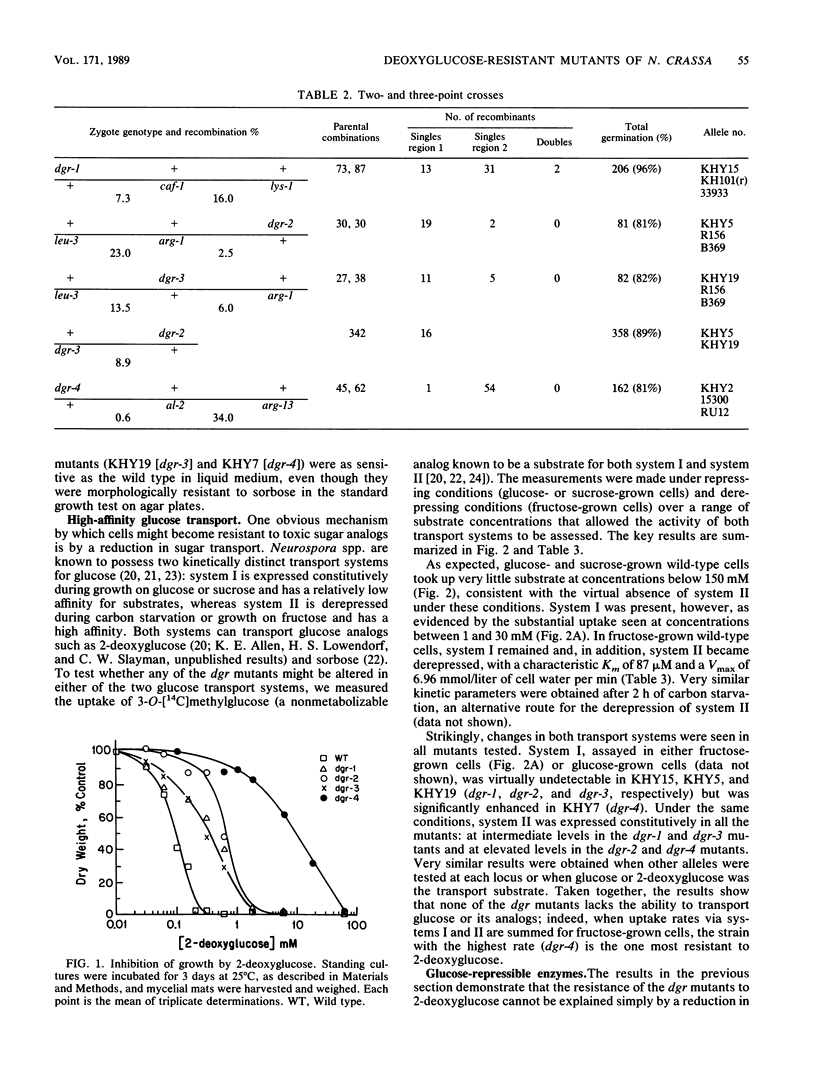
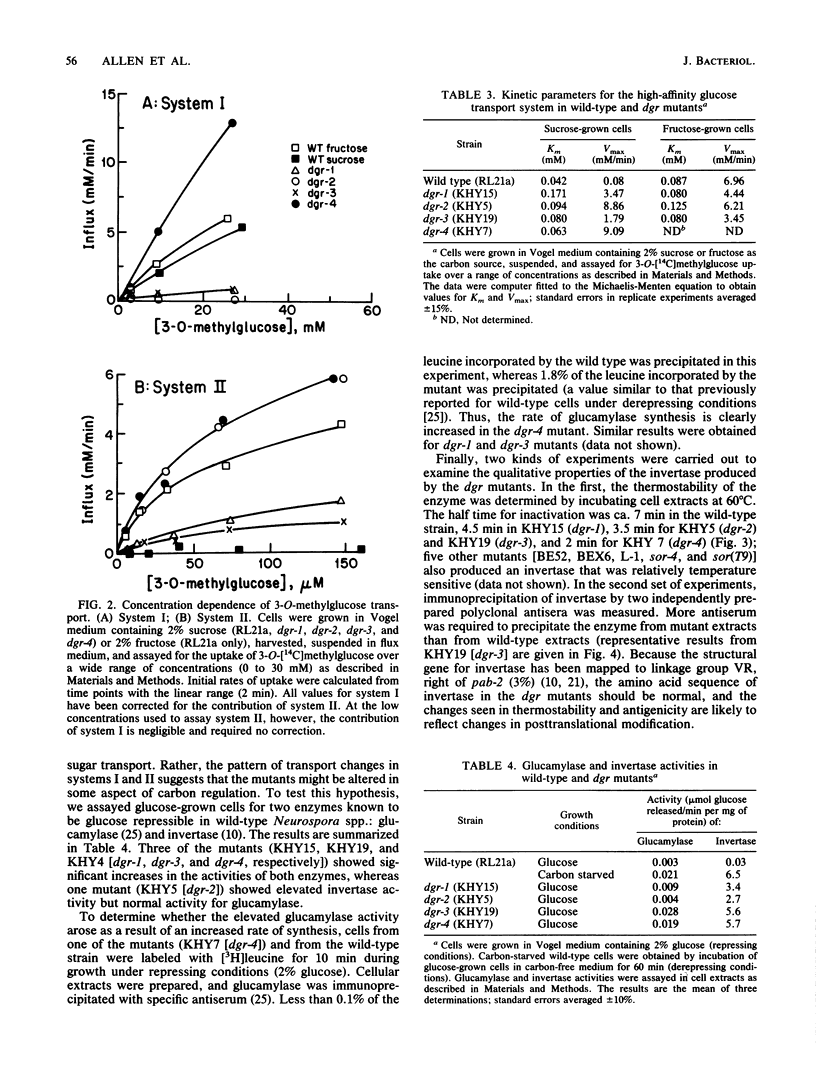
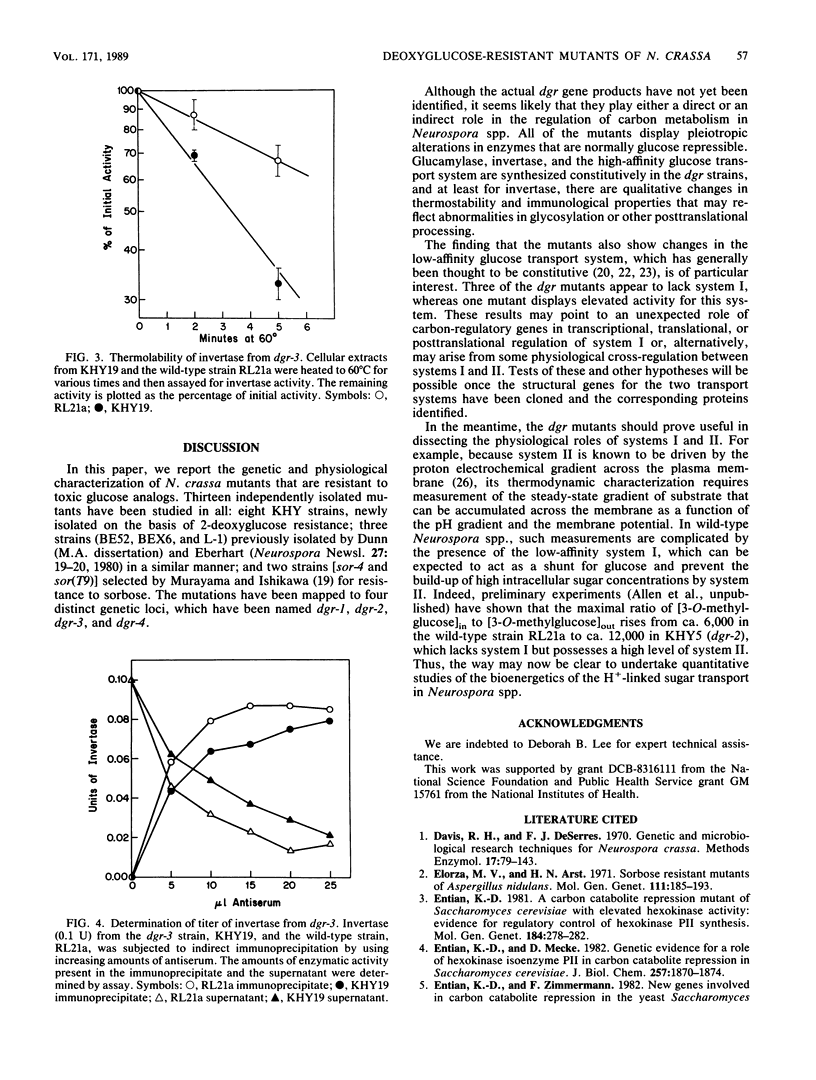
Neurospora crassa mutants resistant to 2-deoxyglucose have been isolated, and their mutations have been mapped to four genetic loci. The mutants have the following characteristics: (i) they are resistant to sorbose as well as to 2-deoxyglucose; (ii) they are partially or completely constitutive for glucose transport system II, glucamylase, and invertase, which are usually repressed during growth on glucose; and (iii) they synthesize an invertase with abnormal thermostability and immunological properties, suggesting altered posttranslational modification. All of these characteristics could arise from defects in the regulation of carbon metabolism. In addition, mutants with mutations at three of the loci lack glucose transport system I, which is normally synthesized constitutively by wild-type N. crassa. Although the basis for this change is not yet clear, the mutants provide a way of studying the high-affinity system II uncomplicated by the presence of the low-affinity system I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elorza M. V., Arst H. N., Jr Sorbose resistant mutants of Aspergillus nidulans. Mol Gen Genet. 1971;111(2):185–193. doi: 10.1007/BF00267792. [DOI] [PubMed] [Google Scholar]
- Entian K. D. A carbon catabolite repression mutant of Saccharomyces cerevisiae with elevated hexokinase activity: evidence for regulatory control of hexokinase PII synthesis. Mol Gen Genet. 1981;184(2):278–282. doi: 10.1007/BF00272917. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K., Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphyorylation. Mol Gen Genet. 1977 Nov 4;156(1):99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K., Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphyorylation. Mol Gen Genet. 1977 Nov 4;156(1):99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- HEREDIA C. F., SOLS A. METABOLIC STUDIES WITH 2-DEOXYHEXOSES. II. RESISTANCE TO 2- DEOXYGLUCOSE IN A YEAST MUTANT. Biochim Biophys Acta. 1964 May 11;86:224–228. doi: 10.1016/0304-4165(64)90046-7. [DOI] [PubMed] [Google Scholar]
- Klingmüller W. Kreuzungs-Analyse Sorbose-resistenter Mutanten von Neurospora crassa. Mol Gen Genet. 1967;100(2):109–116. doi: 10.1007/BF00333598. [DOI] [PubMed] [Google Scholar]
- Lee D. B., Free S. J. Isolation and characterization of Neurospora mutants affected in invertase synthesis. Genetics. 1984 Apr;106(4):591–599. doi: 10.1093/genetics/106.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Resistance to 2-deoxyglucose in yeast: a direct selection of mutants lacking glucose-phosphorylating enzymes. Mol Gen Genet. 1977 Dec 9;157(3):297–300. doi: 10.1007/BF00268666. [DOI] [PubMed] [Google Scholar]
- Maitra P. K. A glucokinase from Saccharomyces cerevisiae. J Biol Chem. 1970 May 10;245(9):2423–2431. [PubMed] [Google Scholar]
- Martin M., Heredia C. F. Characterization of a phosphatase specific for 2-deoxyglucose-6-phosphate in a yeast mutant. FEBS Lett. 1977 Nov 15;83(2):245–248. doi: 10.1016/0014-5793(77)81014-4. [DOI] [PubMed] [Google Scholar]
- Moore D., Devadatham M. S. Sugar transport in Coprinus cinereus. Biochim Biophys Acta. 1979 Feb 2;550(3):515–526. doi: 10.1016/0005-2736(79)90153-6. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. Structure of the capsular and extracellular polysaccharides of Rhizobium japonicum that bind soybean lectin. J Biol Chem. 1982 Feb 25;257(4):1870–1875. [PubMed] [Google Scholar]
- Murayama T., Ishikawa T. Mutation in Neurospora crasa affecting some of the extracellular enzymes and several growth characteristics. J Bacteriol. 1973 Sep;115(3):796–804. doi: 10.1128/jb.115.3.796-804.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Suskind S. R., Roseman S. A derepressible active transport system for glucose in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1294–1301. [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Gene-enzyme relationships in Neurospora invertase. J Bacteriol. 1969 Feb;97(2):867–872. doi: 10.1128/jb.97.2.867-872.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. II. A second glucose transport system. J Biol Chem. 1970 Aug 10;245(15):3985–3987. [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Kinetic characteristics of the two glucose transport systems in Neurospora crassa. J Bacteriol. 1971 May;106(2):479–486. doi: 10.1128/jb.106.2.479-486.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Regulation of sugar transport in Neurospora crassa. J Bacteriol. 1971 May;106(2):487–492. doi: 10.1128/jb.106.2.487-492.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund R. D., McNally M. T., Lee D. B., Free S. J. Neurospora glucamylase and a mutant affected in its regulation. Biochem Genet. 1985 Feb;23(1-2):89–103. doi: 10.1007/BF00499115. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]



