Abstract
Aim
To determine the prevalence and risk factors associated with secondary glaucoma postcongenital cataract surgery.
Methods
All children diagnosed as having congenital cataracts in a major children's hospital between 1985 and 2005 were included in a retrospective case series. Medical records of 423 eyes among 283 patients who underwent cataract surgery with or without intraocular lens implantation at age ⩽16 for congenital cataract were reviewed. The main outcome measure was presence or absence of secondary glaucoma and time to glaucoma postsurgery. The following risk factors were evaluated: age at cataract surgery, presence of systemic anomalies, microcornea, persistent hyperplastic primary vitreous (PHPV), primary capsulotomy/anterior vitrectomy, primary intraocular lens implantation, secondary membrane surgery and duration of postoperative observation.
Results
The statistical methods were the use of Kaplan–Meier survival analysis and Multivariate Cox hazards regression analysis. The mean follow‐up was 6.3 (SD 5.0) years (median 4.6 years; range 0.5 to 20.3 years). Glaucoma developed in 36 of 234 patients (15.4%). Multivariate Cox proportional hazards regression analysis identified age less than 9 months at time of surgery (RR 2.9, 95% CI 1.3 to 7.7; p = 0.03), microcornea (RR 3.7, 95% CI 2.0 to 7.0; p<0.001), and follow‐up time as important predictors of glaucoma. PHPV (RR 1.4, 95% CI 0.7 to 2.7; p = 0.41) and primary posterior capsulotomy/anterior vitrectomy (RR 2.2, 95% CI 0.9 to 5.5; p = 0.17) were not significantly associated with secondary glaucoma in the multivariate model. The mean time to glaucoma after congenital cataract surgery was 4.9 years (range 2 weeks to 16.8 years).
Conclusion
Secondary glaucoma is an important sequela in patients who undergo surgery for congenital cataracts. It is imperative that these patients get lifelong surveillance, as glaucoma can occur years after the initial operation.
Secondary glaucoma is an important postoperative complication of paediatric cataract surgery. The incidence has been variably reported as between 6% and 26% in the literature, depending on the length of postoperative follow‐up.1,2,3,4,5,6,7,8 The incidence of secondary glaucoma postpaediatric cataract surgery remains high, despite newer surgical techniques. Prior to 1970, the incidence was high due to needling of the cataracts.9 With the introduction of automated lensectomy and vitrectomy, it was hoped that by removing the lens and the capsular remnants more effectively, postoperative inflammation would be reduced and thereby reduce the incidence of secondary glaucoma.10,11,12,13,14,15,16
Aphakic glaucoma in these children is hard to diagnose and treat. These children can remain asymptomatic, despite the high intraocular pressure (IOP). As the onset of paediatric aphakic glaucoma is delayed, the signs of glaucoma in infancy including epiphora, blepharospasm, photophobia, increasing corneal diameter, Haab's striae and corneal clouding may not be seen. Further, it is difficult to examine these children due to young age, poor fundal view due to posterior capsular opacification or nystagmus. Secondary glaucoma postcongenital cataract surgery is very difficult to manage. Two‐thirds of children need 3 or more medications to control their IOP. There is a high failure rate with trabeculectomy, with 50% requiring two or more surgeries to control their IOP. Ultimately, these children may need tube implants to control IOP.17 The purpose of this study was to determine the incidence and risk factors associated with secondary glaucoma postcongenital cataract surgery in an Australian cohort of children from a larger tertiary referral centre.
Materials and methods
The study was a retrospective case series. The study was conducted in accordance with the recommendations of the Declaration of Helsinki and was approved by the Children's Hospital at Westmead Human Ethics Committee. The medical records of consecutive patients with congenital cataracts who had surgery at age 16 years or younger between 1985 and 2005 with or without intraocular lens (IOL) implantation at The Children's Hospital at Westmead were included in this review. To be included in the study, the minimum required follow‐up from the time of surgery to the last examination including IOP measurement was 6 months. Excluded from this review were patients with anterior segment dysgenesis, syndromes associated with glaucoma such as Lowe syndrome, history of ocular trauma, congenital glaucoma or prior ocular surgery other than cataract extraction.
Data were collected from patient notes for risk factors that were considered to be important and relevant to progression to glaucoma. These included age at cataract surgery; family history of cataract or glaucoma; and microcornea, defined as <10 mm of corneal diameter or <9.5 in the first month of life. These criteria were chosen, as they represent corneal diameters at least 1 mm less than the age‐adjusted average for most ages.18,19 Not all patients had a corneal measurement in the patient file, but a clinician's diagnosis of microocornea was taken at face value: persistent hyperplastic primary vitreous (PHPV); systemic anomalies; primary posterior capsulotomy and anterior vitrectomy; secondary membrane surgery; and primary IOL implantation. Data regarding the morphological classification of the cataracts were not available in all patients and are not included in the study. Follow‐up data of these patients were from either The Children's Hospital at Westmead (CHW) or private consulting rooms of the CHW paediatric ophthalmologists.
The main outcome measure was absence or presence of glaucoma and the time to glaucoma from initial operation. Glaucoma was defined as IOP ⩾26 mm Hg as measured by applantation tonometer, Puff tonometry or Tonopen either in the clinic or at induction under general anaesthetic. Optic nerve, gonioscopic and visual field evaluations were not used for definition of glaucoma, as they were not uniformly present in the records of all study subjects. The threshold of 26 mm Hg was chosen in this study to increase the likelihood that identified “glaucoma” patients indeed had glaucoma.18
We used the Statistical Analysis System (SAS; v9; SAS Institute, Cary, NC) for data analyses. Multivariate Cox proportional hazards regression modelling was used to test potential predictors to secondary glaucoma. Univariate models were initially performed to test individual predictors and were subsequently entered in the multivariate model for predictors with p<0.25 in a forward stepwise manner. The criterion for retention in the multivariate model was p<0.05. We used Cox's regression model to estimate proportional hazards ratio. To address potential genetic bias in children with bilateral cataracts developing aphakic glaucoma, an adjustment for potential intrasubject correlation in the Cox models was made using the robust sandwich variance estimate.20 Survival estimates for each cohort was estimated using the Kaplan Meier method.
Results
In this retrospective case series, 423 eyes among 283 patients had surgery performed for congenital cataract over the 20‐year time period (1985–2005). There were 49 patients (17%) who had follow‐up of less than 6 months and were excluded from further analysis. The remaining 234 patients (358 eyes) had adequate follow‐up for inclusion in the study. Of these, 110 had a unilateral cataract, and 124 had bilateral cataracts.
Glaucoma was diagnosed at a mean of 4.9 (SD 2.2) years postcataract surgery (median, 3.6 years; range, 10 days to 16.8 years). At follow‐up, 48 study eyes had developed glaucoma, which is 13.4% of eyes, and 36 of the 234 patients had developed glaucoma, which is 15.4% of patients. The mean postcataract surgery follow‐up to the last examination was 6.3 (5.0) years (median, 4.6 years; range, 0.5 to 20.3 years) for all study subjects; it was 10.3 (6.9) years (median, 7.8 years; range, 1.5 to 20.3 years) for those who developed glaucoma and 5.7 (4.4) years (median, 4.3 years; range, 0.5 to 20.3 years) for those who did not develop glaucoma (p<0.001).
The mean age at cataract surgery was 2.4 (SD 3.3) years (median, 0.6 years; range, 7 days to 15.9 years) for all study subjects; it was 0.48 (1.1) years (median, 0.21 years; range, 7 days to 5.5 years) for those who developed glaucoma and 2.6 (3.4) years (median, 0.7 years; range, 10 days to 15.9 years) for those who did not develop glaucoma (p<0.001). Descriptive data for study patients' eyes with occurrence of postoperative glaucoma are presented in table 1. Of the children who had bilateral cataracts, 16% of operated eyes developed glaucoma, and of those children with unilateral cataracts, 13% of operated eyes developed glaucoma. Of those with bilateral cataracts, 12/18 (67%) developed glaucoma in both eyes, where one third developed glaucoma in only one eye. Interestingly, none of the patients with bilateral cataracts had glaucoma in the unoperated eye.
Table 1 Descriptive characteristics of subjects with secondary glaucoma.
| Characteristics | Total | No. of eyes with glaucoma (%) |
|---|---|---|
| Sex | ||
| Male | 182 | 19 (10.4) |
| Female | 176 | 29 (16.4) |
| Eye | ||
| Right | 182 | 25 (13.7) |
| Left | 176 | 23 (13.1) |
| Laterality of cataract | ||
| Bilateral | 248 | 30 (12.9) |
| Unilateral | 110 | 18 (16.3) |
Tables 2 and 3 present the univariate and the multivariate Cox proportional hazards model analysis of the potential predictors of secondary glaucoma respectively. The continuous variable age at cataract surgery was analysed in the Cox proportional hazards model both as a continuous variable and as a dichotomous variable (age <9 months or age ⩾9 months). Both models were appropriate, but it was decided to use age as a dichotomous variable in keeping with prior literature.17
Table 2 Univariate Cox Proportional Hazards Ratio of potential predictors to secondary glaucoma with adjustment for intrasubject correlation.
| Risk factor | Hazard ratio (95% CI) | p value |
|---|---|---|
| Female sex | 1.6 (0.9 to 2.8) | p = 0.13 |
| Family history of congenital glaucoma or cataracts | 2.2 (1.2 to 3.9) | p = 0.01 |
| Microcornea | 5.4 (3.0 to 9.7) | p<0.001 |
| PHPV | 3.4 (1.8 to 6.3) | p<0.001 |
| Systemic anomaly | 1.3 (0.6 to 2.6) | p = 0.49 |
| Primary posterior capsulotomy and anterior vitrectomy | 4.5 (1.9 to 10.5) | p<0.001 |
| Age at cataract surgery less than 9 months | 6.0 (2.4 to 15.2) | p<0.001 |
| Secondary membrane surgery | 1.2 (0.7 to 2.2) | p = 0.54 |
Table 3 Multivariate Cox Proportional Hazards Ratio of potential predictors to secondary glaucoma with adjustment for intrasubject correlation.
| Risk factor | Hazard ratio (95% CI) | p value |
|---|---|---|
| Family history of congenital glaucoma or cataracts | 2.5 (1.4 to 4.6) | p = 0.002 |
| Microcornea | 3.7 (2.0 to 7.0) | p<0.001 |
| PHPV | 1.4 (0.7 to 2.7) | p = 0.41 |
| Primary posterior capsulotomy and anterior vitrectomy | 2.2 (0.9 to 5.5) | p = 0.17 |
| Age at cataract surgery less than 9 months | 2.9 (1.3 to 7.7) | p = 0.03 |
Primary IOL implantation was performed in 96 eyes, and none of these patients developed secondary glaucoma. Table 4 compares the mean ages, follow‐up duration and time to glaucoma between the pseudophakic and aphakic eyes.
Table 4 Comparison of eyes in children who underwent surgery for cataract with or without primary intraocular lens implant.
| Pseudophakic eyes | Aphakic eyes | p value | |
|---|---|---|---|
| No. of eyes | 96 | 261 | |
| Glaucoma | 0 | 48 | |
| Glaucoma diagnosis, postop years (SD) | NA | 4.9 (2.2) | |
| Age at surgery, years (SD) | 5.9 (3.5) | 1.1 (2.1) | p<0.001 |
| Follow‐up time, years (SD) | 4.2 (3.3) | 7.0 (5.3) | p<0.001 |
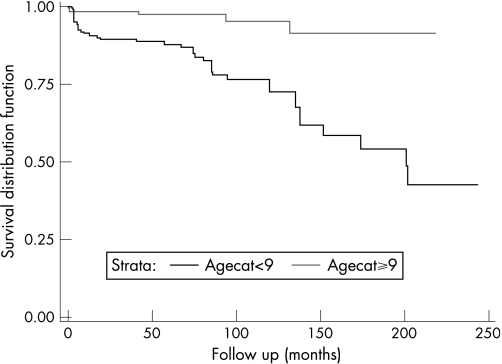
The survival curve for the relationship between age at cataract surgery and the development of secondary glaucoma is shown in fig 1. Survival analysis predicts higher rates of glaucoma with a longer duration of follow‐up. The 10‐year prevalence rate of glaucoma is 29.9% in the group “age at cataract surgery 9 months or less”; this compares with 5% in the group “age at cataract surgery greater than 9 months”. This difference is more pronounced at the 20‐year follow‐up. The prevalence rate of glaucoma is 57.0% in the group “age at cataract surgery 9 months or less”; this compares with 8.8% in the group “age at cataract surgery greater than 9 months”.
Figure 1 Survival curve for the relationship between age at cataract surgery and the development of secondary glaucoma as estimated from the multivariate Cox proportional hazards model.
The median logMAR visual acuity for the study population was 0.5. The proportion of subjects with logMAR visual acuity >0.5 (visual acuity less than 6/18) was 48.5% for those who did not develop glaucoma and 86.5% for those who did develop glaucoma.
Discussion
Congenital cataracts have a devastating visual consequences and account for 10% of childhood blindness worldwide. The timing of surgery for cataracts depends on the morphology of the cataract, and early surgery is aimed to prevent irreversible amblyopia. However, early intervention surgery is also significantly associated with postoperative glaucoma. Our study was conducted on a group of paediatric cataract patients with surgery performed using modern techniques. The definition of glaucoma based on IOP rather than the use of optic nerve, visual field and gonioscopic findings is consistent with other paediatric glaucoma studies.6,8,21 It is acknowledged that the true prevalence of glaucoma in these children varies, depending on the definition used to define glaucoma. The inclusion of optic nerve changes in patients with IOP <26 mm Hg would increase the true prevalence of secondary glaucoma. However, central corneal thickness, which is increased in aphakic/pseudophakic children, may decrease the true prevalence of glaucoma, as the IOP may be overestimated with increased central corneal thickness.22
The frequency of secondary glaucoma in paediatric cataract surgery varies, depending on the length of the follow‐up. Our study identified a prevalence of 13.4% for secondary glaucoma. These results are consistent with previous published literature, 6–26%.4,5,6,8,18,21,23,24,25,26 The minimum follow‐up of 6 months for inclusion into the study may underestimate the true prevalence, as a longer follow‐up is associated with increased prevalence of secondary glaucoma.
The onset of secondary glaucoma can occur over a long period of time. In our study, the mean time to glaucoma was 4.9 years which is consistent with other reports, 2.6 to 12.2 years.4,5,6,8,19,21,23,25,26 Secondary glaucoma can occur years after the initial operation; the range in this series was 10 days to 16.8 years, highlighting the need for lifelong surveillance of these children.
Age at cataract surgery is a known predictor of progression to secondary glaucoma. Various thresholds for age have been documented in the literature ranging from 10 days to 5 years of life.3,4,6,19,24,25,27,28,29,30,31 A recent paper by Rabiah in the JAAPOS hypothesised a threshold of age at 9 months to be a significant predictor to glaucoma: those operated on or before the age of 9 months were 3.8 times likely to progress to glaucoma than those who were operated after the age of 9 months.18 Our data support this theory, with age less than 9 months a significant predictor to progression to glaucoma. In our cohort of patients, those children who were operated on at an age of 9 months or less were three times more likely to progress to glaucoma than those who were operated on after the age of 9 months. At the end of the study period, the prevalence rate of glaucoma was 57.0% in the children who were operated on at an age of 9 months or less.
The study also confirmed microcornea as a significant risk factor in progression to glaucoma. In this study, the diagnosis of microcornea was made by the clinician; however, precise diagnosis of microcornea using a caliper would avoid measurement bias. Our study identified family history of congenital cataract or glaucoma as a predictor to secondary glaucoma. However, due to the nature of the data collection, we are unable to differentiate whether this effect was due to a family history of congenital cataract or family history of glaucoma. The role of genetics in secondary glaucoma needs further evaluation.
Primary posterior capsulotomy/anterior vitrectomy and PHPV have been documented in previous studies to be important predictors to secondary glaucoma.1,2,4,21 In our study, both these factors were significant predictors of progression to secondary glaucoma in the univariate model. However, they did not reach statistical significance in the multivariate models. This is likely due to a correlation between the explanatory variables; most patients who had a PHPV or a primary posterior capsulotomy/anterior vitrectomy were also likely to be very young (age less than 9 months) at the time of cataract surgery. We acknowledge that PHPV and primary posterior capsulotomy/anterior vitrectomy are recognised risk factors to progression to glaucoma in previous published literature, and our lack of statistical significance in the multivariate model may reflect the sample size and the nature of inherent bias in retrospective studies.
Various theories have been hypothesised with regard to the mechanism by which secondary glaucoma in these children develops. There are two theories that have gained significant momentum. One suggests that the filtration angle of the infant eye is susceptible to postoperative inflammation.20 The second theory relates to the mechanical absence of a lens which somehow induces aphakic glaucoma.8 Asrani et al identified over 1000 cases of primary IOL in congenital cataract surgery and identified only one case of secondary glaucoma. They proposed that the IOL prevents toxic vitreous metabolites entering the anterior chamber or that support for the trabecular meshwork is lost in aphakia.23 It is interesting to note that in our case series, none of the 96 patients who had a primary IOL had glaucoma. This suggests that intraocular lenses in some way are protective against glaucoma. However, these results need to be interpreted with caution, as the eyes selected for primary IOL may be considered “normal”, and eyes predisposed to secondary glaucoma may be excluded from primary IOL implantation. In our cohort, only 4 patients had an IOL implanted prior to the age of 9 months. A recent retrospective review by Trivedi and colleagues suggests that selection bias is the reason for the observed low incidence of glaucoma in pseudophakia. In their review for patients who underwent cataract surgery in the first 4.5 months of life, the glaucoma incidence was 24% for children with pseudophakic eyes and 19% for aphakic eyes.32 The Infant Aphakia Treatment Study has a secondary aim of examining complications, including the development of glaucoma in aphakic children.33 This may then be able to provide an answer to what extent pseudophakia is protective against glaucoma in these children.
This study confirms the high prevalence of secondary glaucoma after congenital cataract surgery and that the variable nature of onset for secondary glaucoma requires lifelong surveillance for these children. Further prospective studies are warranted to discover the role of primary IOL in prevention of secondary glaucoma and to uncover the pathogenesis of this visually debilitating disease.
Contributions of authors
BS and JG designed and conducted the study; BS collected and managed the data; BS, JG, FB and FM analysed and interpreted the data; BS prepared the manuscript; and JG, FM, FB, CD, SH, JS and RJ reviewed and approved the manuscript.
Abbreviations
IOL - intraocular lens
IOP - intraocular pressure
PHPV - persistent hyperplastic primary vitreous
Footnotes
Funding/support: Nil.
Competing interests: None declared.
References
- 1.Chrousos G A, Parks M M, O'Neill J F. Incidence of chronic glaucoma, retinal detachment and secondary membrane surgery in pediatric aphakic patients. Ophthalmology 1984911238–1241. [DOI] [PubMed] [Google Scholar]
- 2.Egbert J E, Wright M M, Dahlhauser K F.et al A prospective study of ocular hypertension and glaucoma after pediatric cataract surgery. Ophthalmology 19951021098–1101. [DOI] [PubMed] [Google Scholar]
- 3.Keech R V, Tongue A C, Scott W E. Complications after surgery for congenital and infantile cataracts. Am J Ophthalmol 1989108136–141. [DOI] [PubMed] [Google Scholar]
- 4.Mills M D, Robb R M. Glaucoma following childhood cataract surgery. J Pediatr Ophthalmol Strabismus 199431355–360. [DOI] [PubMed] [Google Scholar]
- 5.Miyahara S, Amino K, Tanihara H. Glaucoma secondary to pars plana lensectomy for congenital cataract. Graefes Arch Clin Exp Ophthalmol 2002240176–179. [DOI] [PubMed] [Google Scholar]
- 6.Parks M M, Johnson D A, Reed G W. Long‐term visual results and complications in children with aphakia. A function of cataract type. Ophthalmology 1993100826–840. [DOI] [PubMed] [Google Scholar]
- 7.Robb R M, Petersen R A. Outcome of treatment for bilateral congenital cataracts. Ophthalmic Surg 199223650–656. [PubMed] [Google Scholar]
- 8.Simon J W, Mehta N, Simmons S T.et al Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology 199198670–674. [DOI] [PubMed] [Google Scholar]
- 9.Francois J. Late results of congenital cataract surgery. Ophthalmology 1979861586–1598. [DOI] [PubMed] [Google Scholar]
- 10.Billson F A, Tarr K H. Closed system intraocular surgery in young people. Trans Ophthalmol Soc N Z 19813371–76. [PubMed] [Google Scholar]
- 11.Fink A I, McGroarty J F. Use of the ocutome in anterior segment surgery. Dev Ophthalmol 1981577–83. [DOI] [PubMed] [Google Scholar]
- 12.Parks M M. Intracapsular aspiration. Int Ophthalmol Clin 19771759–74. [PubMed] [Google Scholar]
- 13.Parks M M. Visual results in aphakic children. Am J Ophthalmol 198294441–449. [DOI] [PubMed] [Google Scholar]
- 14.Parks M M. Posterior lens capsulectomy during primary cataract surgery in children. Ophthalmology 198390344–345. [DOI] [PubMed] [Google Scholar]
- 15.Taylor D. Choice of surgical technique in the management of congenital cataract. Trans Ophthalmol Soc U K 1981101114–117. [PubMed] [Google Scholar]
- 16.Mandal A K, Netland P A. Glaucoma in aphakia and pseudophakia after congenital cataract surgery. Indian J Ophthalmol 200452185–198. [PubMed] [Google Scholar]
- 17.Bhola R, Keech R V, Olson R J.et al Long‐term outcome of pediatric aphakic glaucoma. J AAPOS 200610243–248. [DOI] [PubMed] [Google Scholar]
- 18.Rabiah P K. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol 200413730–37. [DOI] [PubMed] [Google Scholar]
- 19.Wallace D K, Plager D A. Corneal diameter in childhood aphakic glaucoma.[see comment]. J Pediatr Ophthalmol Strabismus 199633230–234. [DOI] [PubMed] [Google Scholar]
- 20.Lin D Y, Wei L J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989841074–1078. [Google Scholar]
- 21.Johnson C P, Keech R V. Prevalence of glaucoma after surgery for PHPV and infantile cataracts. J Pediatr Ophthalmol Strabismus 19963314–17. [DOI] [PubMed] [Google Scholar]
- 22.Simon J W, O'Malley M R, Gandham S B.et al Central corneal thickness and glaucoma in aphakic and pseudophakic children. J AAPOS 20059326–329. [DOI] [PubMed] [Google Scholar]
- 23.Asrani S, Freedman S, Hasselblad V.et al Does primary intraocular lens implantation prevent “aphakic” glaucoma in children? J AAPOS 2000433–39. [DOI] [PubMed] [Google Scholar]
- 24.Lundvall A, Zetterstrom C. Complications after early surgery for congenital cataracts. Acta Ophthalmol Scand 199977677–680. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson G, Abrahamsson M, Sjostrand J. Glaucoma following congenital cataract surgery: an 18‐year longitudinal follow‐up. Acta Ophthalmol Scand 20007865–70. [DOI] [PubMed] [Google Scholar]
- 26.Taylor R H, Ainsworth J R, Evans A R.et al The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS 19993308–315. [DOI] [PubMed] [Google Scholar]
- 27.Chen T C, Walton D S, Bhatia L S. Aphakic glaucoma after congenital cataract surgery. Arch Ophthal 20041221819–1825. [DOI] [PubMed] [Google Scholar]
- 28.Vishwanath M, Cheong‐Leen R, Taylor D.et al Is early surgery for congenital cataract a risk factor for glaucoma?[see comment]. Br J Ophthalmol 200488905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts P, Abdolell M, Levin A V. Complications in infants undergoing surgery for congenital cataract in the first 12 weeks of life: is early surgery better? J AAPOS 2003781–85. [DOI] [PubMed] [Google Scholar]
- 30.Pressman S H, Crouch E R., Jr Pediatric aphakic glaucoma. Ann Ophthalmol 198315568–573. [PubMed] [Google Scholar]
- 31.Walton D S. Pediatric aphakic glaucoma: a study of 65 patients. Trans Am Ophthalmol Soc 199593403–413. [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi R H, Wilson M E, Jr, Golub R L. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. J AAPOS 200610117–123. [DOI] [PubMed] [Google Scholar]
- 33. The infant aphakia treatment study. http://clinicaltrials.gov/ct/show/NCT00212134;jsessionid = 9E680506267EDA66C43 D361469E2BC48 order = 8 (accessed 20 September)