Hypoxia‐inducible factors (HIF‐1α and HIF‐2α) play pivotal roles in angiogenesis. However, their involvement in choroidal neovascular membranes (CNVMs) is still unknown. This study investigates the distribution of HIF‐1α and HIF‐2α proteins in six CNVMs associated with age‐related macular degeneration (AMD). By means of immunohistochemical analysis, HIF‐1α and HIF‐2α were detected in 5 and 6 eyes, respectively. Endothelial cells and macrophages were immunostained by both HIF‐1α and HIF‐2α antibodies, whereas no staining was observed in retinal pigment epithelial (RPE) cells. Our study raises the possibility that HIF‐1α and HIF‐2α are involved in CNV formation.
HIF‐1α and HIF‐2α are transcription factors that transactivate the expression of pro‐angiogenic genes in response to hypoxic conditions, and play important roles in vasculogenesis and angiogenesis.1,2 HIF‐1α is increased in ischaemic retina, subsequently upregulating the expression of vascular endothelial growth factor (VEGF).3 HIF‐2α exerts pro‐angiogenic functions in retinopathy of prematurity, presumably by upregulating the expression of a potent angiogenic factor, erythropoietin.4 However, to the best of our knowledge, there has been no study investigating the expression of HIF proteins in human CNVMs. In this study, we performed immunohistochemical analysis to investigate the distribution of HIF proteins in CNVMs.
Specimens were obtained from 6 eyes of 6 AMD patients (aged 65–86 years). Informed consent for the use of excised tissue was obtained from all patients. All procedures followed the tenets of the Declaration of Helsinki, and Institutional Review Board approval was obtained for the study. Between January 2003 and September 2004, submacular surgery was performed according to the technique described previously.5 Fluorescein angiography was used to classify the CNV type.6 Clinical characteristics of all patients are presented in table 1.
Table 1 Clinical characteristics.
| Eye | Age | Sex | Affected eye | CNV type6 classification | CNVM size* |
|---|---|---|---|---|---|
| 1 | 80 | M | R | Classic | 1.5 |
| 2 | 79 | F | L | Predominantly classic | 0.8 |
| 3 | 79 | F | L | Classic | 1.5 |
| 4 | 65 | M | L | Classic | 1.0 |
| 5 | 86 | M | R | Classic | 1.3 |
| 6 | 83 | F | L | Classic | 1.0 |
M, Male; F, Female; R, right; L, left
CNV, choroidal neovascularization; CNVM, choroidal neovascular membrane
*in disc diameter
Each CNVM specimen was immediately embedded in OCT compound (O.C.T. Compound; Sakura Finetek Co., Ltd., Tokyo, Japan), rapidly frozen and prepared for avidin‐Biotin complex immunohistochemistry (VECTASTAIN ABC kit, Vector Laboratories, Burlingane, CA).
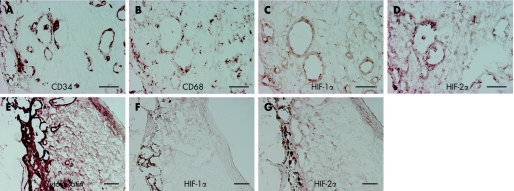
HIF‐1α and HIF‐2α were detected in 5 and 6 eyes of 6 eyes, respectively, as revealed by immunolabelling with anti‐HIF‐1α and anti‐HIF‐2α antibody (Novus Biologicals, Littleton, CO). Neovascular vessels immunostained by CD34 (Biomeda, Foaster, CA) were detected in all CNVMs and located in stroma, and half of the endothelial cells were immunostained by both HIF proteins (fig 1A‐B). CD68‐positive (Ylem, Roma, Italy) macrophages were located uniformly in stroma in all CNVMs and almost all macrophages were stained with both HIF antibodies (fig 1C‐D). All specimens contained a partially intact monolayer of RPE cells located on one side of the CNVMs and immunostained by pancytokeratin (Sigma, St. Louis, MO) (fig 1E‐F). No overlap was observed with HIF proteins and RPE cells (fig 1E‐F). The distribution pattern of HIF‐1α and HIF‐2α in the CNVMs was similar in all specimens.
Figure 1 Immunostaining of CNVMs by cell‐specific marker and HIF. Immunostaining was performed to confirm the cellular source of expression for HIF‐1α and HIF‐2α. (A–D) Serial sections were immunostained by CD34, CD68, HIF‐1α and HIF‐2α. The vascular endothelium cells were stained by CD34 (A), and some of the endothelial cells were stained by HIF‐1α and similarly by HIF‐2α. (C and D) Macrophages, identified by immunostaining of CD68 (B), were detected abundantly in all CNVMs and were located uniformly in stroma. Most of the macrophages that were immunostained were immunoreactive to HIF‐1α and HIF‐2α (C and D). A partially intact monolayer of retinal pigment epithelium (RPE) cells located on one side of the CNVMs was immunostained by pancytokeratin (E). However, no overlap was observed with HIFs staining (F and G) and RPE cells. Scale bars, 50 μm.
In summary, HIF‐1α and HIF‐2α are detected in the endothelium and macrophages. The HIF transcription factors activate the expression of the VEGF gene in response to hypoxic conditions. VEGF, in turn, induces angiogenesis.7,8 RPE cells, endothelial cells and macrophages are VEGF‐positive in CNVMs from AMD patients,9,10 suggesting that VEGF is involved in CNVM formation.10 Our results, together with previous results, may indicate that HIFs in endothelial cells induce VEGF, thereby contributing to the development of CNVM. In the current study, however, expression of HIF proteins was not detectable in RPE cells, raising the possibility that other mechanisms are involved in the production of VEGF in these cells. Further studies are needed to explore the possible causal relationship between hypoxia and the expressions of HIF proteins.
Footnotes
Competing interests: None declared
References
- 1.Wang G L, Jiang B H, Rue E A.et al Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐pas heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995925510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pugh C W, Ratcliffe P J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med 20039677–684. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki H, Yu A Y, Della N.et al Hypoxia inducible factor‐1alpha is increased in ischemic retina: Temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 199940182–189. [PubMed] [Google Scholar]
- 4.Morita M, Ohneda O, Yamashita T.et al HIF/HIF‐2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. Embo J 2003221134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert H M, Capone A, Jr, Aaberg T M.et al Surgical excision of subfoveal neovascular membranes in age‐related macular degeneration. Am J Ophthalmol 1992113257–262. [DOI] [PubMed] [Google Scholar]
- 6.Barbazetto I, Burdan A, Bressler N M.et al Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment – TAP and VIP report No.2. Arch Ophthalmol 20031211253–1268. [DOI] [PubMed] [Google Scholar]
- 7.Leung D W, Cachianes G, Kuang W J.et al Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 19892461306–1309. [DOI] [PubMed] [Google Scholar]
- 8.Kim K J, Li B, Winer J.et al Inhibition of vascular endothelial growth factor‐induced angiogenesis suppresses tumour growth in vivo. Nature 1993362841–844. [DOI] [PubMed] [Google Scholar]
- 9.Frank R N, Amin R H, Eliott D.et al Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol 1996122393–403. [DOI] [PubMed] [Google Scholar]
- 10.Oh H, Takagi H, Takagi C.et al The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1999401891–1898. [PubMed] [Google Scholar]