Abstract
Aim
To compare the pupil signs in patients with bilateral pupillotonia caused by Holmes–Adie syndrome or generalised peripheral neuropathy.
Methods
Infrared video pupillographic techniques were used to measure a number of pupil variables in patients with Holmes–Adie syndrome, generalised neuropathy (various aetiologies) and healthy age‐matched control subjects.
Results
Regardless of aetiology, the patients generally had pupil signs typical of pupillotonia (small dark diameters, large light diameters, tonic near responses, attenuated light responses with light‐near dissociation, and sector palsy). However, significant differences were found in the prevalence and magnitude of several pupil variables in the two patient groups. In particular, sector palsy and anisocoria exceeding 1 mm (in the light) were seen much more commonly in Holmes–Adie patients than patients with generalised neuropathy. The presence of both these pupil signs can be used to distinguish between these diagnoses with a sensitivity of 58% and a specificity of 90%.
Conclusions
The tonic pupils of patients with Holmes–Adie syndrome are significantly different to those found in patients with generalised neuropathy; recognition of these differences may allow distinction between these diagnoses.
Holmes–Adie syndrome (HAS)1,2 is an idiopathic condition occurring most commonly in young women. The typical pupil findings include attenuation of the light response, sector palsy,3 a “tonic” near response (large amplitude but slow velocity miosis on accommodative effort) and denervation supersensitivity to dilute muscarinic receptor agonists. In addition, patients have reduced tendon jerks and, in some cases, patchy hypohidrosis (a variant known as Ross syndrome4). Although careful testing reveals mild subclinical autonomic disturbances elsewhere in the body in some patients,5 HAS remains a benign condition, and patients do not go on to develop signs of a more generalised peripheral neuropathy.
When an otherwise healthy patient presents with an unilateral tonic pupil and tendon areflexia, the diagnosis of HAS is usually straightforward. However, bilateral tonic pupils with areflexia may be part of HAS6 but may also be seen in patients with generalised peripheral or autonomic neuropathy,7 and it can be difficult to establish the aetiology. The purpose of this study was therefore to determine whether there are any differences in the pupil examination between the tonic pupils associated with Adie/Ross syndromes and the tonic pupils caused by peripheral neuropathies. Preliminary results from this study have been presented to the European Neuro‐Ophthalmological Society.8
Methods
Subjects
The pupillographic records of 140 consecutive patients referred to us with bilateral tonic pupils have been examined. Sixty‐one of these (21 male; 40 female; age range 18–70 years) were diagnosed as having Adie's syndrome (50) or Ross syndrome (11) on clinical grounds based on typical pupil findings, tendon areflexia and absence of any other neurological symptoms or signs as discussed above. The remaining 79 patients (33 male; 46 female; age range 11–80 years) had generalised neuropathic disorders diagnosed according to standard clinical criteria and supported by appropriate investigations including nerve conduction studies, autonomic function tests, nerve biopsies, serology and neuro‐genetics. Cases seen included patients with: amyloidosis (8), diabetes mellitus (15), Charcot–Marie‐Tooth disease (12), acute or chronic dysautonomia (10), Sjögren's syndrome (4), Triple A syndrome (4), paraneoplastic syndromes (4) and miscellaneous other peripheral neuropathies (22).
Pupil assessment
Pupil diameters in the dark (DD) and in bright light (DL), and the amplitude of the pupillary response to a standard intensity light flash (“light response”, LR) or a standardised accommodative task (“near response”, NR) were recorded by infrared video pupillometry. The presence or absence of sector palsy was judged clinically on slit‐lamp examination, and local pathology within the iris or anterior segment of the eye excluded.
Statistics
Pupil diameters and anisocoria estimates in the dark and in the light were compared with normative data from age‐matched healthy control subjects.7 Light‐near dissociation (LND) was confirmed if the amplitude of the near response (NR) exceeded that of the light response (LR). Differences between the continuous variables (age of patient, pupil diameter, anisocoria, LR, NR) were compared using Student's t test (or the Mann–Whitney rank sum test, if standard normality criteria were not satisfied). Differences between numbers observed with categorical variables (gender, presence or absence of light‐near dissociation or sector palsy) were compared using χ2 tests with Yates's correction for continuity (or Fisher's exact test if numbers were too small). The usefulness of anisocoria measurements in discriminating between Adie/Ross syndromes and generalised neuropathy was evaluated by constructing a receiver‐operating characteristic curve9 showing the variation of sensitivity/specificity pairs across the entire range of decision thresholds.
Results
Age and gender distribution
The demographics of all patients and healthy subjects included in this study are summarised in table 1. No significant differences were found in age or gender distribution between the Adie/Ross patients compared with patients with generalised neuropathy. However, a subgroup analysis comparing Adie patients with Ross patients revealed that Ross patients were significantly older than Adie patients (t = −2.275; p = 0.027) and more commonly male (p = 0.036 using Fisher's exact test). The healthy control subjects were reasonably age‐matched to both patient groups, but because the majority of patients were female, the gender distribution was significantly different in the control group.
Table 1 Age and gender distribution of patient and control groups.
| Diagnosis | N | M | F | Median age | Age range | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| Adie | 50 | 14 | 36 | 40.5 | 18 | 70 |
| Ross | 11 | 7 | 4 | 50 | 33 | 65 |
| Neuropathy | 79 | 33 | 46 | 51 | 11 | 80 |
| Controls | 315 | 172 | 143 | 42 | 16 | 82 |
Pupil diameter
Measurements of pupil diameters in the dark and in the light are summarised in table 2. Not all measurements were available in every patient (numbers shown), and for the purposes of this table, the average pupil diameter was compared with that expected from age‐matched controls. The results show that in darkness, pupil diameters were generally smaller than expected on the basis of age in both the Adie/Ross (−1.54 (0.17) mm) and the neuropathy (−1.22 (0.15) mm) groups, but the difference between the two groups was not significant (t = 1.412, p = 0.160). In contrast, under bright lighting conditions, pupil diameters were larger than expected in both the Adie/Ross (+1.62 (0.11) mm) and the neuropathy (+1.19 (0.11) mm) groups. This departure of light diameter measurements from expected was significantly greater in the Adie/Ross patients compared with the neuropathy patients (t = 2.543, p = 0.013). Sub‐group analysis considering dark and light diameters only of the smaller pupil or of the larger pupil in each patient revealed similar results.
Table 2 Comparison of pupil measurements in patients with Adie/Ross syndrome and generalised neuropathy.
| DD (O–E) | DL (O–E) | LR | NR | LND | |
|---|---|---|---|---|---|
| Adie/Ross | |||||
| N | 61 | 34 | 58 | 53 | 53 |
| Mean | −1.54 | +1.62 | 9% | 26% | −17% |
| SEM | 0.17 | 0.11 | 1% | 1% | 2% |
| Neuropathy | |||||
| N | 78 | 56 | 75 | 66 | 65 |
| Mean | −1.22 | +1.19 | 15% | 23% | −7% |
| SEM | 0.15 | 0.11 | 1% | 1% | 2% |
| Comparison | |||||
| t | 1.432 | 2.543 | 3.319 | 1.629 | 3.901 |
| P | 0.160 | 0.013 | 0.001 | 0.106 | <0.001 |
For each group, the mean difference (mm) between the pupil diameter (average of two eyes) observed in the patients and that expected from age‐matched control subjects, both in the dark (DD) and in the light (DL), are shown together with the standard error of the mean (SEM). The mean response amplitude to a standard intensity light stimulus (LR) or near effort (NR) is given as a percentage of baseline pupil diameter, and the degree of light‐near dissociation (LND = LR–NR) derived. Measurements in Adie/Ross and neuropathy patients have been compared using Student's t test, and significant differences are shown in bold.
Responses to light and near
Averaged measurements (from both eyes) of the pupil responses to light (LR) and to near (NR), expressed as percentage miosis, are shown in table 2. Both groups of patients showed attenuated light responses when compared with controls, but the light responses were significantly smaller in the Adie/Ross patients compared with those with generalised neuropathy (t = 3.319; p = 0.001). The near responses were generally normal in amplitude (albeit tonic) in all patients, and there was no significant difference between the groups. Light‐near dissociation (defined as NR>LR) was more commonly observed in Adie/Ross patients (43/53 cases) than in neuropathy patients (31/65 cases) (χ2 = 12.567; p<0.001). Moreover, the degree of light‐near dissociation (LND) was greater in Adie/Ross patients compared with neuropathy patients (t = 3.901; p<0.001).
Anisocoria
Among the healthy control subjects, the 95% upper limits to anisocoria in the dark and in the light were 0.7 mm and 0.5 mm, respectively. Anisocoria measurements lying outside these normal limits were found more frequently in Adie/Ross patients (30/61 cases in the dark; 25/34 cases in the light) compared with neuropathy patients (17/78 cases in the dark; 17/56 cases in the light). Chi‐squared tests confirm the significance of these different proportions (in darkness: χ2 = 10.280; p = 0.001; in light: χ2 = 14.156; p<0.001).
Moreover, measurements of the degree of anisocoria were also different between these patient groups (see table 3), patients with Adie/Ross syndrome showing significantly more anisocoria than the neuropathy patients.
Table 3 Anisocoria measurements (mm) in the dark and in the light in patients with Adie/Ross syndrome or generalised neuropathy.
| Anisocoria: | Dark (mm) | Light (mm) |
|---|---|---|
| Adie/Ross | ||
| N | 61 | 34 |
| Mean | 1.00 | 1.25 |
| SEM | 0.12 | 0.18 |
| Neuropathy | ||
| N | 78 | 56 |
| Mean | 0.44 | 0.45 |
| SEM | 0.05 | 0.05 |
| Comparison | ||
| t | 4.697 | 5.218 |
| P | <0.001 | <0.001 |
N, number of patients. Anisocoria measurements in Adie/Ross and neuropathy patients were compared using Student's t test, and significant differences are shown in bold.
Presence of sector palsy
On slit‐lamp biomicroscopy, the majority of patients in both groups showed sector palsy, but the proportion of Adie/Ross patients (32/35 or 91.4%) was significantly higher than the proportion of neuropathy patients (27/39 or 69.2%) (χ2 = 4.334; p = 0.037).
Identifying which pupil measurement variables best distinguish these diagnostic groups
Some of the pupil variables measured in this study are significantly different in the two patient groups. To assess the capacity for any variable to distinguish between these diagnoses, we have chosen arbitrary thresholds for the continuous variables and evaluated the proportions of both patient groups exceeding these thresholds, allowing estimation of the sensitivity and specificity of each variable as a test to distinguish between the two aetiologies. The results are shown in table 4.
Table 4 Capacity of different pupil variables (alone and combined) to discriminate Adie/Ross (A/R) from neuropathy (Neuro) patients.
| Variable | Threshold* | A/R | Neuro | χ2 | P | Sens (%) | Spec (%) |
|---|---|---|---|---|---|---|---|
| DL (O–E) | >+1.4 mm | 20/34 | 23/56 | 2.672 | 0.102 | 58.8 | 58.9 |
| AL | >1 mm | 18/34 | 7/56 | 17.247 | <0.001 | 52.9 | 87.5 |
| LR | <12.2% | 40/58 | 38/75 | 4.516 | 0.034 | 69.0 | 49.3 |
| NR | <24.4% | 30/53 | 41/66 | 0.372 | 0.542 | 56.6 | 37.9 |
| LND | <11.9% | 29/53 | 22/65 | 5.182 | 0.023 | 54.7 | 66.2 |
| SP | Present | 32/35 | 27/39 | 5.624 | 0.018 | 91.4 | 30.8 |
| AL+SP | As above | 14/24 | 3/29 | 11.765 | <0.001 | 58.3 | 89.7 |
| AL+SP+LND | 6/20 | 2/26 | 2.517 | 0.113 | 30.0 | 92.3 |
*Arbitrary thresholds giving maximum discrimination between patient groups.
DL (O–E), deviation from expected diameter in the light; AL, anisocoria in the light; LR, light response; NR, near response; LND, light‐near dissociation; SP, sector palsy. Sens, sensitivity; spec, specificity.
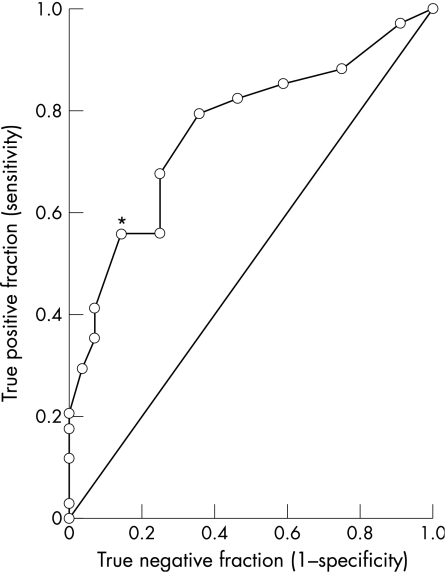
In most cases χ2 tests confirmed that the pupil variables and thresholds chosen resulted in significantly different proportions in the two patient groups. The variable associated with the greatest sensitivity (ie, ability to identify patients with pupillotonia caused by Adie/Ross syndrome) was the presence of sector palsy (91.4%). The variable giving the greatest specificity (ie, best at identifying patients with pupillotonia not caused by Adie/Ross syndrome) was anisocoria less than 1 mm in the light (87.5%). The interplay between sensitivity and specificity across a wide range of arbitrary thresholds for anisocoria is displayed on the receiver‐operating characteristic plot shown in fig 1. Anisocoria in the light is seen to discriminate well between these two patient groups at all thresholds. Even greater specificity was achieved by combining two or three of these pupil variables: the presence of sector palsy and light anisocoria exceeding 1 mm was rarely seen in neuropathy patients (3/29 cases), giving a specificity of 89.7%. Addition of LND further increased specificity to 92.3%, but reduced the sensitivity of the test to 30.0%.
Figure 1 Nonparametric receiver‐operating characteristic (ROC) plot for anisocoria measurements in the light to distinguish between Adie/Ross and neuropathic aetiologies for bilateral pupillotonia. The line of equality indicates the theoretical plot for a test with no power to discriminate between these diagnoses. A test with perfect discrimination would give an ROC plot that passes through the upper left corner. *Sensitivity/specificity pair corresponding to the arbitrary threshold used in table 4 (1 mm).
Discussion
In this study, we have compared the pupil findings in patients with bilateral pupillotonia associated with either Adie/Ross syndrome or generalised neuropathies. Many of the findings were similar in the two groups. However, both the prevalence and the degree of some of these abnormalities varied significantly. The clearest difference found was in the degree of anisocoria, particularly in the light: patients with Adie/Ross syndrome are much more likely to have significant anisocoria compared with patients with a generalised neuropathy. We suggest that the explanation for this may lie in the different natural histories of these conditions. Adie/Ross syndrome typically affects one pupil first, with the other pupil becoming involved often years later; since the pupils then progressively miose, this sequential damage gives rise to marked anisocoria in contrast to patients with generalised neuropathy where it is likely that both pupils are involved simultaneously.
The difference in some of these pupil variables could in theory allow the ophthalmologist to distinguish between pupillotonia associated with Adie/Ross syndrome and that caused by a more generalised neuropathy. In practice, there is overlap between the patient populations for all the measurements studied, so no single variable absolutely discriminates between the two diagnoses. From a clinical perspective, the most useful pupil variables to make this diagnostic distinction are those that are easily measured and do not require comparison with a normal dataset. Moreover, tests with high specificity are more relevant than those with high sensitivity (Adie/Ross syndromes are relatively common; what the ophthalmologist needs is a test that helps to exclude the rarer but less benign cases of widespread neuropathy).
On the basis of our results, the best combination of pupil variables for distinguishing between the two diagnoses is the presence of sector palsy and light anisocoria >1 mm. These pupil signs are easily assessed by any ophthalmologist, and if both are present the clinician can conclude that the pupil abnormality is very unlikely to be due to a generalised neuropathy (specificity 90%), even if such a neuropathy is actually present. Conversely, if both are absent, then generalised neuropathy must be considered. If one or other is present, then the test has limited capacity to discriminate between the diagnoses.
A common situation where such a distinction is important is when a patient with diabetes is referred to the ophthalmologist with asymptomatic bilateral pupillotonia. The important clinical question is whether the pupillotonia is caused by widespread diabetic autonomic neuropathy (DAN), which carries a grave prognosis,10 or whether it is associated with the unrelated and benign Holmes–Adie syndrome (HAS). The prevalence of pupillotonia caused by DAN or HAS in the diabetic population is not known, but an intelligent guess at the aetiology can be made by determining whether or not there is sector palsy or light anisocoria >1 mm: none of the patients with DAN in this study had both features, and if present, the clinical diagnosis is more likely to be HAS.
Acknowledgements
We are grateful to the British Eye Research Foundation whose grant enabled us to build the pupillographic equipment used in this study.
Abbreviations
DAN - diabetic autonomic neuropathy
HAS - Holmes–Adie syndrome
LND - light‐near dissociation
LR - light response
NR - near response
Footnotes
Competing interests: None.
References
- 1.Holmes G. Partial iridoplegia associated with symptoms of other diseases of the nervous system. Trans Ophthal Soc UK 193151209–228. [Google Scholar]
- 2.Adie W J. Tonic pupils and absent tendon reflexes: a benign disorder sui generis. Its complete and incomplete forms. Brain 19325598–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson H S. Segmental palsy of the iris sphincter in Adie's syndrome. Arch Ophthal 1978961615–1620. [DOI] [PubMed] [Google Scholar]
- 4.Ross A T. Progressive selective sudomotor degeneration. Neurology 19588809–817. [DOI] [PubMed] [Google Scholar]
- 5.Bacon P J, Smith S E. Cardiovascular and sweating dysfunction in patients with Holmes–Adie syndrome. J Neurol Neurosurg Psych 1993561096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson H S, Adie's syndrome: some new observations Trans Am Ophthal Soc. 1977;75:587–626. [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner F D, Smith S E. Pupil findings in a consecutive series of 150 cases of generalised autonomic neuropathy. J Neurol Neurosurg Psych 2006771163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremner F D, Smith S E. Bilateral tonic pupils. Neuro‐Ophthalmol 200327219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zweig M H, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 199339561–577. [PubMed] [Google Scholar]
- 10.Ewing D J, Campbell I W, Clarke B F. The natural history of diabetic autonomic neuropathy. Quart J Med 19804995–108. [PubMed] [Google Scholar]