Abstract
Aim
To report the early vitreous involvement in a rare familial amyloidotic polyneuropathy (FAP) mutation and associated vitreous vascular endothelial growth factor (VEGF) levels.
Design
Observational case series.
Methods
Review of clinical, pathological, photographic,
and angiographic records of two FAP siblings with severe vitreous involvement. Laboratory ELISA analysis of vitreous samples for VEGF, and DNA sequence analysis of peripheral blood for transthyretin (TTR) mutational analysis.
Results
Two patients underwent 25‐gauge vitrectomy in three eyes with marked improvement of visual acuity. Neovascularisation seen intraoperatively responded to endolaser. Analysis of vitrectomy samples for VEGF showed raised levels in all three specimens. Mutational analysis revealed an isolated Glu54Gly mutation in the transthyretin gene.
Conclusions
Early involvement of the vitreous occurs in a rare transthyretin mutation of FAP, with increased vitreous levels of VEGF.
Keywords: retina, amyloid, VEGF
Familial amyloidotic polyneuropathy (FAP) comprises a genetically heterogeneous group of disorders characterised by amyloid deposition within peripheral nerves, kidneys, and cardiac muscle, as well as within the eye. Transmission is autosomal dominant with most mutations—of which there have been more than 80 described—affecting the transthyretin (TTR) gene.1 The presence of retinal neovascularisation and vitreous haemorrhage have been described previously in these patients, along with documentation of amyloid deposition within the walls of retinal and choroidal vessels.2 This led to the hypotheses that retinal ischaemia and the potential weakening of vessel walls caused by the deposition of the amyloid were aetiological factors behind the observed retinal vascular abnormalities. To investigate this further we analysed undiluted vitreous samples, obtained during pars plana vitrectomy, for vascular endothelial growth factor (VEGF) in these two patients with a rare form of FAP associated with early vitreous involvement.
Case reports
Case 1
A 32 year old Korean man presented with a two year complaint of decreased vision and floaters, worse in the right eye than in the left. His family history was positive for FAP in four of six family members. He had undergone orthotopic liver transplant 10 months before presentation. Visual acuities measured counting fingers at 1 foot in the right eye (OD) and 20/50 left eye (OS), with normal intraocular pressures. Slit lamp examination was significant for bilateral eyelid ecchymoses. Fundus examination after pupillary dilatation revealed 4+ vitreous opacities with a glass‐wool appearance, which obscured the view to the posterior pole in the right eye, and 2+ vitreous opacities in the left eye (fig 1). Vitrectomy using 25‐gauge instrumentation was carried out in the right eye. Endolaser was applied to areas of neovascularisation intraoperatively (fig 2). By one week postoperatively, visual acuity had improved to 20/30 in the right eye. Four months later the patient presented with a new complaint of acutely decreased vision in the left eye. Visual acuity was counting fingers at 2 feet. Vitreous haemorrhage with associated glass wool opacities was found on dilated examination (fig 1). A 25‐gauge vitrectomy with endolaser treatment was carried out on the left eye. Visual acuity stabilised at 20/30 by one week postoperatively.
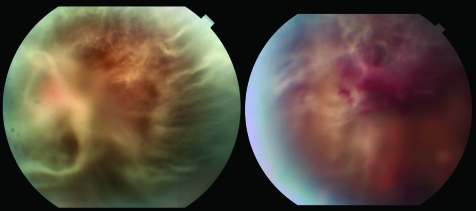
Figure 1 Left: Fundus photograph of the right eye of patient 1 before vitrectomy, showing severe vitreous amyloid deposition. Right: Preoperative fundus photograph of the left eye of patient 1 with evidence of vitreous haemorrhage.
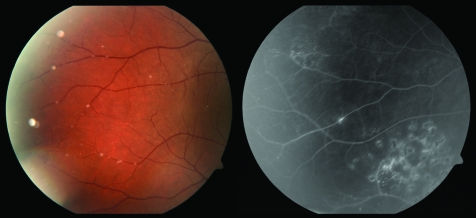
Figure 2 Left: Postoperative colour fundus photograph of the right eye of patient 1, illustrating areas of retinal vascular abnormality as well as fine deposits of amyloid associated with retinal vessels. Right: Fluorescein angiogram of the same eye showing areas of endolaser and a small tuft of neovascularisation.
Case 2
A 30 year old Korean man, the sibling of patient 1, presented with a three year history of bilateral floaters with progressive blurring of vision. He had been seen one year earlier and diagnosed as having vitreous amyloidosis. He had undergone orthotopic liver transplantation eight months previously. Visual acuities were 20/50 OD, 20/200 OS, with normal intraocular pressures. Slit lamp examination showed bilateral ecchymoses of the upper lids. Fundus examination of the right eye after pupillary dilatation revealed focal areas of glass‐wool appearing vitreous deposits, some of which seemed to emanate from the blood vessels, as well as mid‐peripheral dot–blot haemorrhages. The left eye had similar intraretinal haemorrhages and a large vitreous opacity obscuring the nerve and most of the macula.
Vitrectomy with 25‐gauge instruments was carried out in the left eye. Endolaser was applied to areas of neovascularisation identified intraoperatively. The postoperative course was complicated by mild hypotony and shallow, peripheral serous choroidal effusions which resolved by postoperative week 3. Visual acuity improved to 20/70 in the left eye and was limited by an epiretinal membrane.
Pathology
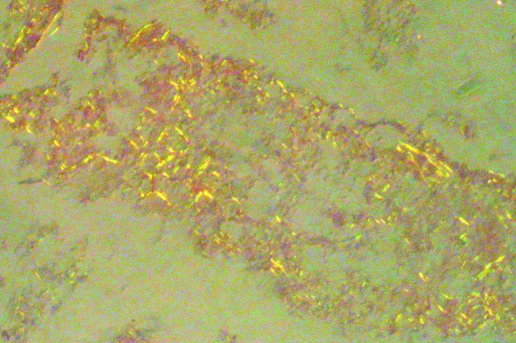
Pathological analysis of all vitreous aspirates showed staining with Congo Red and the characteristic yellow‐green birefringence seen with amyloid deposits (fig 3). Peripheral blood was obtained from patient 1 and was sent for genetic analysis. Patient 2 had returned to Korea before consenting to analysis. Mutational analysis carried out on peripheral blood from patient 1 (Mayo Medical Laboratories) revealed an isolated Glu54Gly transthyretin mutation (DNA change: 221 A→G). Enzyme linked immunosorbent assay (ELISA) analysis for VEGF was undertaken on undiluted vitrectomy specimens obtained at the time of vitrectomy for vitreous opacities (R&D Systems, Quantikine, Minneapolis, Minnesota, USA). The VEGF levels were significantly raised in all three eyes. Patient 1 had VEGF concentrations of 854 pg/ml in the right eye and 623 pg/ml in the left eye; patient 2 had a VEGF concentration of 360 pg/ml in the left eye. Analysis of a separate control sample from a patient who underwent PPV for a macular hole revealed a VEGF level of 128 pg/ml.
Figure 3 Histological features of an undiluted vitrectomy specimen stained with Congo Red and illuminated with polarised light, showing yellow‐green birefringence characteristic of amyloid deposits.
Discussion
To our knowledge this is the first documentation of increased vitreous VEGF levels in the setting of FAP. Some studies have suggested that the haemorrhages associated with FAP might be secondary to a weakening of the vessel wall as it is infiltrated with amyloid.3 Retinal neovascularisation, although previously reported, represents an uncommon complication of FAP. In a cohort of 37 patients with FAP caused by a Val30Met mutation with long term follow up, only one developed retinal neovascularisation.4 The levels of VEGF found within the vitreous samples of our FAP patients are raised to a level similar to that found in patients with inactive proliferative diabetic retinopathy (mean 698.2 pg/ml), and consistent with the presence of ischaemia within the posterior segment of these eyes.5
Recent studies of undiluted vitreous specimens from patients with non‐ischaemic ocular diseases, such as macular holes and epiretinal membranes in the absence of proliferative vitreoretinopathy, have found vitreous VEGF levels ranging from undetectable (defined as less than 62.4 pg/ml) to 109 pg/ml.5,6 Thus the markedly raised VEGF levels in our FAP patients suggest that VEGF upregulation and neovascularisation may play a role in the production of haemorrhage. Perhaps amyloid deposition in the retinal vessel walls serves as a barrier to oxygen delivery in the surrounding tissues, thus leading to hypoxia induced upregulation of VEGF.
Isolated Glu54Gly transthyretin mutation therefore represents a very rare TTR mutation. The Glu54Gly transthyretin mutation has been reported twice, once in isolation and once associated with a Gly6Ser transthyretin mutation.7,8 In our two FAP patients, and in the previously reported patient with an isolated Glu54Gly transthyretin mutation, the symptoms began in the late 20s. This is significantly earlier than the age of symptom onset in the reported patients with both the Glu54Gly mutation and the Gly6Ser transthyretin mutation, where vitreous involvement occurred in the middle 40s.7,8
The Gly6Ser transthyretin mutation itself has been described previously in a form of euthyroid hyperthyroxinaemia which is non‐amyloidogenic (no amyloid deposition occurs).9 Thus it had been postulated that the effect of Gly6Ser transthyretin mutation on the TTR structure does not contribute to TTR aggregation and therefore to amyloid deposition. Given the later onset of vitreous involvement in patients with both the Glu54Gly transthyretin mutation and the Gly6Ser mutation, it is possible that the Gly6Ser mutation may even be protective.7 Our two patients with an early onset phenotype in the absence of the Gly6Ser transthyretin mutation add evidence to this hypothesis.
The liver transplants which each patient received corresponded to a subjective decline in vision in both. This was probably secondary to continued amyloid deposition within the vitreous, a phenomenon which has been described previously.10 Although more than 90% of the mutant TTR is produced in the liver, and after liver transplantation its levels in the serum can be reduced to less than 1% of pretransplant levels, it continues to be produced within the eye by the retinal pigment epithelium, thus allowing continued ocular involvement.11 Both patients had chronic visual complaints of floaters and blurred vision several years before their liver transplants, and patient 2 had documented vitreous amyloidosis before transplantation, thus reducing the likelihood that the liver transplant itself was an aetiological factor in their early onset disease.
Conclusions
We report an aggressive FAP phenotype of early, severe vitreous involvement with retinal neovascularisation in the setting of a rare isolated Glu54Gly transthyretin mutation; and we provide what we believe to be the first description of an association between FAP and raised vitreous levels of VEGF, indicating an ischaemic aetiology for the neovascularisation seen in these patients.
Acknowledgements
We thank Bruno Bertoni for his assistance with the images.
Abbreviations
FAP - familial amyloidotic polyneuropathy
TTR - transthyretin
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.Saraiva M J. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat 200117493–503. [DOI] [PubMed] [Google Scholar]
- 2.Kawaji T, Ando Y, Nakamura M.et al Ocular amyloid angiopathy associated with familial amyloidotic polyneuropathy caused by amyloidogenic transthyretin Y114C. Ophthalmology 20051122212. [DOI] [PubMed] [Google Scholar]
- 3.Brett M, Persey M R, Reilly M M.et al Transthyretin Leu12Pro is associated with systemic, neuropathic and leptomeningeal amyloidosis. Brain 1999122183–190. [DOI] [PubMed] [Google Scholar]
- 4.Ando E, Ando Y, Okamura R.et al Ocular manifestations of familial amyloidotic polyneuropathy type I: long‐term follow up. Br J Ophthalmol 199781295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duh E J, Yang H S, Haller J A.et al Vitreous levels of pigment epithelium‐derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol 2004137668–674. [DOI] [PubMed] [Google Scholar]
- 6.Noma H, Minamoto A, Funatsu H.et al Intravitreal levels of vascular endothelial growth factor and interleukin‐6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2006244309–315. [DOI] [PubMed] [Google Scholar]
- 7.Kim H S, Kim S M, Kang S W.et al An aggressive form of familial amyloid polyneuropathy caused by a Glu54Gly mutation in the transthyretin gene. Eur J Neurol 200512657–659. [DOI] [PubMed] [Google Scholar]
- 8.Reilly M M, Adams D, Booth D R.et al Transthyretin gene analysis in European patients with suspected familial amyloid polyneuropathy. Brain 1995118849–856. [DOI] [PubMed] [Google Scholar]
- 9.Fitch N J, Akbari M T, Ramsden D B. An inherited non‐amyloidogenic transthyretin variant, [Ser6]‐TTR, with increased thyroxine‐binding affinity, characterized by DNA sequencing. J Endocrinol 1991129309–313. [DOI] [PubMed] [Google Scholar]
- 10.Ando E, Ando Y, Haraoka K. Ocular amyloid involvement after liver transplantation for polyneuropathy. Ann Intern Med 2001135931–932. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y, Terazaki H, Nakamura M.et al A different amyloid formation mechanism: de novo oculoleptomeningeal amyloid deposits after liver transplantation. Transplantation 200477345–349. [DOI] [PubMed] [Google Scholar]