Abstract
Aim
To describe the different retinal morphological characteristics that can present on optical coherence topography (OCT) in a spectrum of uveitic diseases.
Methods
We reviewed the literature and our own OCT image archive for characteristic features that may be suggestive of a particular disease process.
Results
OCT demonstrates a variety of characteristic morphological changes, some that may point towards a specific disease process. We describe the various forms of macular oedema found in uveitis as well as OCT features typically found in multifocal choroiditis, serpiginous chorioretinitis, toxoplasma chorioretinitis, Vogt–Koyanagi–Harada, sympathetic ophthalmia and the vitreomacular traction syndrome.
Conclusion
Ophthalmologists should be aware of the variety of retinal morphological characteristics that can present on OCT in uveitic disease. Recognition may aid in the diagnostic process, which is complementary to conventional fundal photography and fluorescein angiography. This can facilitate earlier diagnosis and, more importantly, the initiation of specific treatment.
Since its introduction in 1991,1 optical coherence tomography (OCT), has found its place as a widely accepted imaging technique, especially in ophthalmology and other biomedical applications. It represents an interferometric, non‐invasive optical tomographic imaging technique offering millimetre penetration with submicrometre axial and lateral resolution. The technique when first described demonstrated a 30‐µm resolution, but modern tomography such as the introduction of third‐generation commercial OCT instruments (Stratus OCT3, Carl Zeiss Meditec, Dublin, CA) in 2002 made it possible to obtain an axial image resolution of approximately 10 μm. In ophthalmology, OCT makes it possible to obtain noncontact, high‐resolution, cross‐sectional imaging of the retina. This ability of OCT to image tissue morphology in situ and in real time has been termed “optical biopsy”. Quantitative measurement or morphology of the retinal architecture can be used to assess retinal pathology, and the data are then displayed in a false‐colour topographic map. The evaluation of OCT tomograms depends on the observer to identify both differences in the relative reflectivity of different tissue layers and morphological changes in tissue structures.
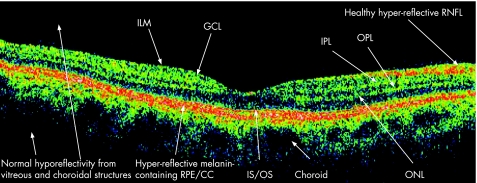
Interpretation of a normal OCT is imperative before pathological disease processes can be appreciated. OCT is able to resolve three highly reflecting layers, believed to correspond to the vitreous/retina, inner/outer photoreceptor segments, and RPE/choriocapillaris interfaces.2 Figure 1 demonstrates an OCT image of the normal human macula. The nerve fibre layer (NFL) is the innermost layer of the retina, followed by the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the photoreceptor inner and outer segments (IS/OS) and the retinal pigment epithelium (RPE). The most backscattering of light is produced from the NFL and the plexiform layers, and can be seen on the OCT image as red or white false‐colour.3
Figure 1 Normal OCT scan.
It is postulated that the reflection emanating from the junction of the inner and outer segments may be caused by the sudden boundary formulated between the structures of the inner, and highly organised outer segments which contains stacks of membranous discs that are rich in the visual pigment rhodopsin.4 OCT imagery of the photoreceptor outer segment supports this interpretation, with the increased reflection being attributed to the increased thickness in the foveal region corresponding to the well‐known increase in the length of the outer cone segments in this region. The melanin‐containing RPE produces strong backscattering and is visualised clearly just below the photoreceptor outer segment. Beneath the RPE, the choriocapillaris and the choroid are visualised as the optical backscattering structure, but unfortunately vascular structures cause light to scatter and limit the penetration of light and the ability of OCT to visualise any of the deeper structures.
Uveitis, although classically associated with the development of macular oedema, can cause a spectrum of retinal morphological changes, some of which characterise specific disease processes. At present, fluorescein angiography is one of the most widely used investigations for detecting the presence of macular oedema. This ancillary test is invasive and certainly not without risk, with approximately 20% of recipients experiencing nausea, with anaphylaxis and death as rare sequelae.5 Furthermore, the information that is provided is qualitative, and its interpretation is highly subjective. Evidence now suggests that OCT is as effective at detecting macular oedema as is fluorescein angiography, but is superior in demonstrating axial distribution of fluid. Compared with flourescein angiography, the OCT sensitivity for detecting cystoid macular oedema was 96%, with a specificity of 100%.6 OCT has been shown to detect macular thickening, even before any angiographic evidence of macular oedema, produces reproducible and consistent results, and provides quantitative measurements of retinal thickness that are ideal for follow‐up and assessment of treatment response to disease.
Macular oedema
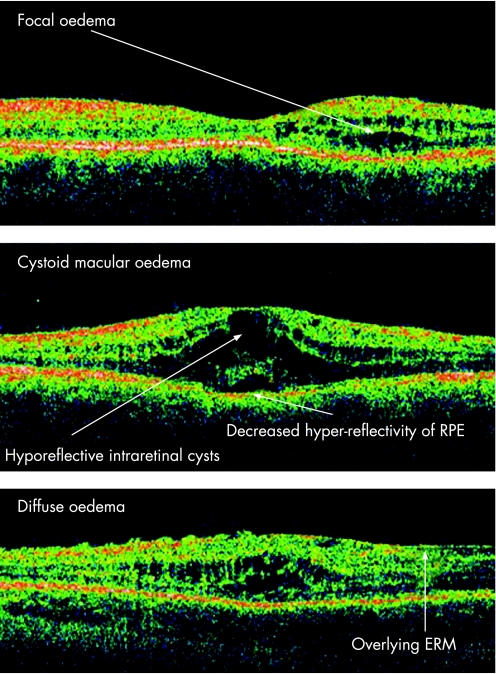
Macular oedema is the leading cause of decreased vision in uveitic patients and can lead to permanent visual impairment in 8.5% of cases.7 Although it may occur with any type of ocular inflammation, it is most commonly associated with pars planitis, iridocyclitis, birdshot retinochoroidopathy, sarcoid uveitis and HLA B27‐associated uveidities. Three patterns of macular oedema are described in uveitis, namely focal, diffuse or cystoid,8 as demonstrated in fig 2.
Figure 2 Patterns of macular oedema.
Although the morphological patterns of diabetic macular oedema are well described,9 it is questionable whether these patterns can be applied to uveitic oedema, as ultrastructural studies suggest possible variations in the morphological features and location of the fluid in different types of macular oedema.10 Pathologically, the oedema is caused by disruption in the normal permeability of the blood–retinal barrier. Inflammatory mediators implicated in promoting barrier breakdown include adenosine, prostaglandin E1, tumour necrosis factor‐α, interleukin‐1‐β, vascular endothelial growth factor and vasoactive peptides. The earlier the detection and treatment of macular oedema, the more favourable the visual prognosis is for the patient as visual acuity improvement is more commonly seen in patients with macular oedema of less than 6 months' duration. The macular changes may be reversible, and restoration of vascular permeability may occur with resolution of the oedema, but with chronic oedema, retinal thinning with photoreceptor damage and progressive fibrosis may occur.11 The accuracy, precision, repeatability and reproducibility of OCT measurements were assessed by Muscat and colleagues, and demonstrated results from control subjects that were repeatable and reproducible with an inter‐session reproducibility of 1.5%.12
White dot syndromes
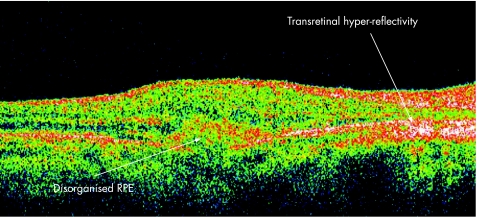
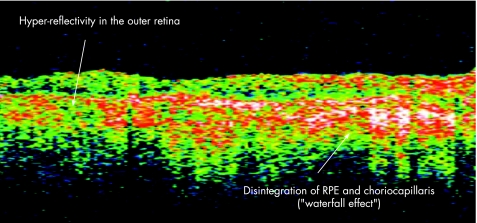
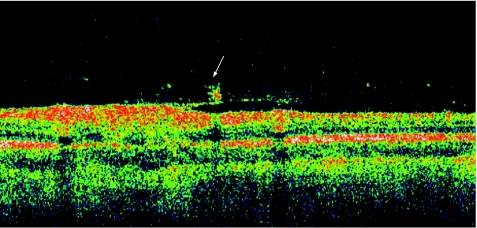
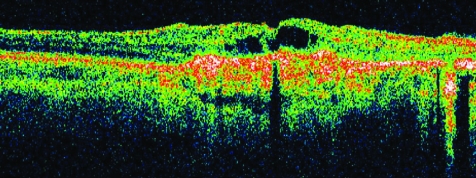
Multifocal choroiditis and panuveitis is a posterior chorioretinal inflammatory disease of unknown aetiology with prominent elements of vitritis and anterior segment uveitis. Subsequent scarring and fibrosis of these lesions with proliferation of the retinal pigment epithelium may lead to the occurrence of choroidal neovascular membranes and possible irreversible visual loss. It has been postulated that the fundal white dots are a clinical presentation of a common abnormality followed by a variable immune‐mediated response causing a spectrum from multiple evanescent white dot syndrome to birdshot chorioretinopathy, with the white dots proposed as microgranulomata.13 Dunlop, in his clinicopathological correlation however, demonstrated that these white dot lesions were non‐granulomatous perivascular choroidal infiltrates consisting mainly of B lymphocytes.14 Van Velthoven and colleagues15 in their paper describe the retinal changes in active multifocal and serpiginous chorioretinitis as seen on OCT. Transretinal hyperreflectivity is the characteristic feature associated with an active multifocal chorioretinal lesion, as is demonstrated in our patient (fig 3). The characteristic feature of an active serpiginous lesion demonstrates hyper‐reflectivity in the outer retina only (fig 4), which correlates well with the histopathology of the disease process. Very few eyes with serpiginous choroiditis have been studied histopathologically, but examination demonstrates extensive loss of the retinal pigment epithelium with destruction of the overlying retina.16 The choriocapillaris as well as part of the choroid is filled with a mononuclear cell infiltrate, suggesting an inflammatory component to this disorder, changes which are represented in this figure as the “Waterfall effect”.
Figure 3 Active multifocal choroiditis and panuveitis.
Figure 4 Active serpiginous choroiditis lesion.
Toxoplasmosis chorioretinitis
The diagnosis of ocular toxoplasmosis is usually based on clinical findings. When the ocular manifestations are atypical, laboratory tests can be supportive, but the diagnosis should not depend solely on serological tests, as the antigen load of a small, active lesion in one eye may not be enough to stimulate elevated systemic antibody titres.17
Several features have been described in association with toxoplasmic chorioretinitis. Guagnini and colleagues in their recent case report18 document the OCT features of spherical deposits associated with recurrent toxoplasmic chorioretinitis. These atypical 100–150‐μm, greyish deposits appear along the retinal arteries and veins as well as on the vitreoretinal interface of the macula. These small granular deposits were originally described by Gass19 and have subsequently been described in human T‐lymphotropic virus type 1‐associated uveitis,20 and in one case of ocular syphilis.21 Our case of acute toxoplasmic chorioretinitis (fig 5) demonstrates these changes with deposits on the vitreoretinal interface with a focal area of increased intraretinal backscatter and thinning of the retina in this area. Furthermore, there is significant backscatter anterior to the retina indicative of the migration of inflammatory cells into the vitreous in association with a secondary partially detached posterior hyaloid face. In contrast, fig 6 demonstrates an OCT scan of an inactive toxoplasmosis lesion. There is a region of enhanced reflectivity within the neurosensory retina corresponding to previous inflammation. There are focal areas of fragmentation within this reflective band corresponding to retinal pigment epithelium proliferation and hyperpigmentation. There is also increased backscatter from the choroid consistent with pigmentary atrophy. The superficial layers of the retina appear to be preferentially involved and thinned, which is consistent with the predilection of toxoplasmosis for neural tissue.
Figure 5 Active toxoplasmosis chorioretinitis lesion. The arrow shows vitreous interface spheroid bodies, partially detached posterior hyaloid face and migration of inflammatory cells into the vitreous.
Figure 6 Inactive toxoplasmosis lesion focal area of fragmentation within the RPE corresponding to RPE proliferation and hyperpigmentation. There is also increased backscatter from the choroid consistent with pigmentary atrophy.
Granulomatous posterior uveitides
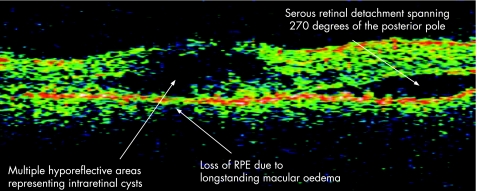
The acute uveitic phase of Vogt–Koyanagi–Harada (VKH) follows the short prodromal phase, with 70% of VKH patients presenting to ophthalmological attention during this phase with bilateral uveitis.22 Early clinical findings include posterior choroidal thickening, manifested as an elevation of the peripapillary retinochoroidal layer, along with disc hyperaemia.23 Subsequent retinal pigment epithelium (RPE) barrier breakdown causes subretinal fluid accumulation and multiple serous retinal detachments. Figure 7 demonstrates the OCT findings in a patient with VKH with multiple intraretinal hyporeflective areas representing intraretinal cysts, degeneration and breakdown of the RPE, and serous retinal detachment. Figure 8 demonstrates bilateral advanced serous macular detachments in a patient with VKH.
Figure 7 Vogt–Koyanagi–Harada syndrome.
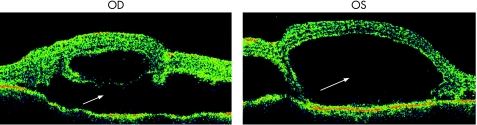
Figure 8 Bilateral serous macular detachments in VKH. Massive amounts of serocus fluid have disrupted the complete inner retinal architecture.
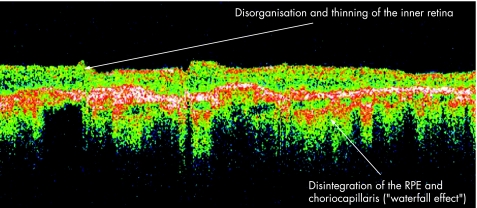
Although much has been said about the histological differences between VKH and sympathetic ophthalmia, it is clear that standard histological studies cannot definitively differentiate one from the other. The histopathologies of the inflammatory changes in sympathetic ophthalmia are identical in the exciting and sympathising eyes.24 Classically, there is sparing of the choriocapillaris, with a relative lack of retinal involvement. There have been, however, atypical histopathological features reported with variable degrees of retinal involvement, including perivasculitis, retinitis, detachment and gliosis.25 Figure 9 demonstrates a patient with sympathetic ophthalmia with disorganisation and thinning of the inner retina, and pronounced disintegration of the RPE and choriocapillaris (“Waterfall effect”).
Figure 9 Sympathetic ophthalmia.
Vitreomacular traction syndrome secondary to idiopathic panuveitis
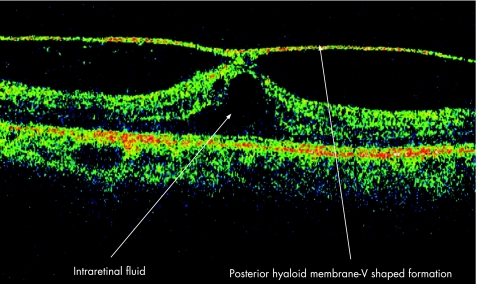
The natural history of vitreomacular traction syndrome (VMT) has been described, and the authors concluded that most symptomatic eyes with VMT underwent a further decrease in visual acuity. Complete vitreomacular separation which occurs infrequently in eyes with the disorder allows resolution of cystoid changes and an improvement of visual acuity.26 The factors responsible for the development of VMT are unknown. Several conditions such as age‐related degeneration and shrinkage of vitreous, metabolic and vascular causes have been described.27 The association of posterior uveitis and VMT is poorly documented. Canzano and colleagues described two cases of VMT following highly active antiretroviral therapy in AIDS patients with cytomegalovirus retinitis, and concluded that changes in immune status may permit an inflammatory response that can lead to VMT.28 Morphologically, two types of vitreous traction can develop in VMT; an incomplete V‐shaped posterior vitreous detachment that leads to foveal retinal detachment, the surgical outcome of which is favourable, and partial posterior vitreous detachment temporal to the fovea in which prominent cystoid macular oedema develops and which may result in a macular hole or macular atrophy postoperatively.29 Figure 10 clearly delineates the characteristic features as seen on OCT. This image represents a V‐shaped posterior vitreal detachment in a patient with idiopathic panuveitis. The posterior hyaloid is hardly distinguishable from the superficial retina on a normal OCT image but becomes apparent when it is detached. This patient would respond favourably to surgical intervention, should the intraretinal oedema persist.
Figure 10 Vitreomacular traction syndrome.
Conclusion
The differential diagnosis of uveitic diseases is largely based on clinical characteristics, a history of prodromal visual and systemic symptoms, signs of inflammation, size and location of lesions, angiographic findings and the course of the disease process. OCT has become a valuable ancillary diagnostic tool, and can provide useful information on the morphological features associated with a variety of uveitic diseases. Ophthalmologists should be aware of these characteristic features so that an earlier diagnosis can be made, and disease‐specific treatments can be instituted.
Abbreviations
OCT - optical coherence tomography
RPE - retinal pigment epithelium
VKH - Vogt–Koyanagi–Harada
VMT - vitreomacular traction syndrome
Footnotes
Funding: None.
Competing interests: None.
References
- 1.Huang D, Swanson E A, Lin C P.et al Optical coherence tomography. Science 19912541178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pons M E, Garcia‐Valenzuela E. Redefining the limit of the outer retina in optical coherence tomography scans. Ophthalmology 20051121079–1085. [DOI] [PubMed] [Google Scholar]
- 3.Toth C A, Narayan D G, Boppart S A.et al A comparison of retinal morphology viewed by optical coherence tomography and by light biomicroscopy. Arch Ophthalmol 19971151425. [DOI] [PubMed] [Google Scholar]
- 4.Hoang Q V, Linsenmeier R A, Chung C K.et al Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci 200219395. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi L A, Rohrer K T, Tindel L J.et al Fluorescein angiography complication survey. Ophthalmology 198693611–617. [DOI] [PubMed] [Google Scholar]
- 6.Antcliff R J, Stanford M R, Chauhan D S.et al Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology 2000107593–599. [DOI] [PubMed] [Google Scholar]
- 7.Malinowski S M, Pulido J S, Folk J C. Long‐term visual outcome and complications associated with pars planitis. Ophthalmology 1993100818–825. [DOI] [PubMed] [Google Scholar]
- 8.Markomichelakis N N, Halkiadakis I, Pantelia E.et al Patterns of macular edema in patients with uveitis. Qualitative and quantitative assessment using optical coherence tomography. Ophthalmology 2004111946–953. [DOI] [PubMed] [Google Scholar]
- 9.Otani T, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol 2000129487–494. [DOI] [PubMed] [Google Scholar]
- 10.Tso M O. Pathology of cystoid macular edema. Ophthalmology 198289902–915. [DOI] [PubMed] [Google Scholar]
- 11.Yu E N. Cystoid macular edema. Uveitis.org. http://www.uveitis.org/medical/articles/case/cme.html (accessed 15 May 2007)
- 12.Muscat S, McKay N, Parks S.et al Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Invest Ophthalmol Vis Sci 2002431791–1795. [PubMed] [Google Scholar]
- 13.Ben Ezra D, Forrester J V. Fundal white dots: the spectrum of a similar pathological process. Br J Ophthalmol 199579856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop A A, Cree I A, Hague S.et al Multifocal choroiditis; clinicopathologic correlation. Arch Ophthalmol 1998116801–803. [DOI] [PubMed] [Google Scholar]
- 15.van Velthoven M E J, Ongkosuwito J V, Verbraak F D.et al Combined en‐face optical coherence tomography and confocal ophthalmoscopy findings in active multifocal and serpiginous chorioretinitis. Am J Ophthalmol 2006141972–975. [DOI] [PubMed] [Google Scholar]
- 16.Wu Is, Lewis H, Fine S L.et al Clinicopathologic findings in a patient with serpiginous choroiditis and treated choroidal neovascularization. Retina 19899292. [DOI] [PubMed] [Google Scholar]
- 17.Pereira‐Da Mata A, Oréfice F. Toxoplasmosis. In: Foster CS, Vitale AT, eds. Diagnosis and treatment of uveitis. Philadelphia: Saunders, 2002385–410.
- 18.Guagnini A P, De Potter P, Levecq L.et al Atypical spherical deposition on vitreoretinal interface associated with toxoplasmic chorioretinitis. Graefe's Arch Clin Exp Ophthalmol 2007245158–160. [DOI] [PubMed] [Google Scholar]
- 19.Gass J M D. Toxoplasmosis retinitis. In: Gass JMD, ed. Stereoscopic atlas of macular diseases: diagnosis and treatment, 4th ed. Philadelphia: Mosby 1997614–622.
- 20.Nakao K, Ohba N. HTLV‐1 associated uveitis revisited: characteristic grey‐white, granular deposits on retinal vessels. Br J Ophthalmol 199680719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crouch E R. Goldberg MF. Retinal periarteritis secondary syphilis. Arch Ophthalmol 197593384–387. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura S. Vogt–Koyanagi–Harada disease. Jpn J Ophthalmol 1978229–35. [Google Scholar]
- 23.Goto H, Rao N A. Sympathetic ophthalmia and Vogt–Koyanagi–Harada syndrome. Int Ophthalmol Clin 199030279–285. [DOI] [PubMed] [Google Scholar]
- 24.Lubin J R, Albert D M, Weinstein M. Sixty‐five years of sympathetic ophthalmia. A clinicopathological review of 105 cases (1913–1978). Ophthalmology 198087109–121. [DOI] [PubMed] [Google Scholar]
- 25.Winter F C. Sympathetic uveitis: A clinical and pathologic study of the visual result. Am J Ophthalmol 195539340–347. [PubMed] [Google Scholar]
- 26.Hikichi T, Yoshida A, Trempe C L. Course of vitreomacular traction syndrome. Am J Ophthalmol 199511955–61. [DOI] [PubMed] [Google Scholar]
- 27.Gass J M D. Vitreous traction maculopathies. In Gass JMD. Stereoscopic atlas of macular diseases: diagnosis and treatment, 4th ed. Philadelphia: Mosby 1997671–725.
- 28.Canzano J C, Reed J B, Morse L S. Vitreomacular traction syndrome following highly active antiretroviral therapy in AIDS patients with cytomegalovirus retinitis. Retina 199818443–447. [DOI] [PubMed] [Google Scholar]
- 29.Yamada N, Kishi S. Tomographic features and surgical outcomes of vitreomacular traction syndrome. Am J Ophthalmol 2005139112–117. [DOI] [PubMed] [Google Scholar]