Abstract
Background
Long‐term outcome and complications of diode laser cyclophotocoagulation (DCPC) may be important, since eyes, once treated with DCPC, are less likely to be subjected to other types of interventions in the further follow‐up.
Methods
Retrospective review of 131 eyes of 127 patients treated from 2000 through 2004. Success was defined as intraocular pressure (IOP) at last visit 6–21 mm Hg; hypotony: IOP ⩽5 mm Hg.
Results
Mean follow‐up (FU) was 30.1 (SD 16.7) months. Mean number of treatment sessions per eye was 1.54, 89% of the eyes having 1 or 2 sessions; overall re‐treatment rate: 38.9%. Mean total laser energy delivered per eye: 133.9 (73.7) J; mean energy per treatment episode: 86.8 (22.0) J. Eyes with 3 or more treatments (11%) had a significantly larger proportion of post‐traumatic glaucoma, and patients were significantly younger. All eyes had refractory glaucomas on maximal medication, neovascular glaucoma (NVG) representing the largest subgroup (61%). IOP decreased from 36.9 (10.7) mm Hg pretreatment to 15.3 (10.4) mm Hg at the end of FU. Success was noted in 69.5% (91 eyes), failure (non‐response) in 13%. Hypotony occurred in 17.6% eyes, of which 74% had NVG. Hypotony developed after mean 19.3 (11.0) months, range 6 to 36; with 96% of these eyes having received only 1 or 2 treatments; delivered energy did not differ from that in the successful eyes.
Conclusions
DCPC is an efficient treatment for refractory glaucoma. Hypotony, the most common complication, may develop as late as 36 months post‐treatment. Diagnostic category and age seem to influence the outcome stronger than laser protocol and delivered energy.
Cyclodestructive procedures have an established place in the management of high‐pressure glaucoma, particularly in advanced cases, refractory to other treatment modalities.1,2,3,4,5 The trans‐scleral cyclophotocoagulation with the diode laser has gained popularity and practically replaced the cryo method, but also the Nd:YAG laser method, due to comparable efficacy and increased safety and tolerability. It is, however, known that the therapeutic effect can be lost over time, and that non‐responsiveness makes repeated treatments necessary in a considerable number of cases.6,7,8,9,10,11,12 Data about dose‐ and laser protocol‐related pressure response and complications vary considerably in the literature. Parameters such as total delivered energy per eye, number of laser burns per session, combination of pulse power and pulse duration, sparing or not sparing a portion of the circumference have been analysed with varying, partially contradictory results.13,14,15 Although several long‐term observations have been published,16,17,18 the majority of the data in the literature refer to mean follow‐up (FU) periods up to 1 year,9,10,11,12,14,15,19,20 which may influence the results. Long‐term aspects of this treatment method may be important, since eyes treated with cyclophotocoagulation are less likely to be subjected to other types of interventions in the further follow‐up.
The purpose of this study was to analyse retrospectively the long‐term safety and efficacy of diode CPC in eyes with refractory glaucoma in a tertiary referral clinical centre.
Methods
A retrospective chart review was performed of all patients that had undergone diode laser cyclophotocoagulation (DCPC) over a period of 5 years (2000–2004) at the Department of Ophthalmology at the University Hospital in Bern, Switzerland. Data were collected about patient demographics, diagnosis, glaucoma medication, intraocular pressure (IOP) course, complications, visual acuity and laser parameters.
DCPC was indicated in refractory cases, when IOP control could not be achieved with maximal tolerated topical and systemic (oral acetazolamide) medication in glaucoma eyes having undergone (or not suitable for) conventional filtering or drainage surgery, and when severely elevated IOP was causing discomfort and pain in terminal glaucoma.
Treatment was conducted under topical (retrobulbar injection of 3.5 ml of 20% mepivacain) or short general anaesthesia using the continuous‐wave semiconductor diode laser (810 nm) and the fibre‐optic Iris G‐probe (OcuLight SLx, IRIS Medical Instruments, Mountain View, CA). Transillumination to localise the ciliary body band was not used routinely but according to surgeon's preference—in approximately 15% of the cases, when ciliary body location was uncertain. The footplate of the G‐probe was placed according to the transillumination results or, routinely, about 0.5 mm behind the limbus, and moderate pressure was exerted. There was no standard treatment protocol. In practically all cases, 270° of the circumference was treated, and either the supero‐temporal or the superior sector (the site of previous surgeries) was spared. Pulse duration was fixed at 2 s. Pulse power and the number of burns varied from case to case and among the surgeons. In general, pulse power was set at 1750 or 2000 mW to start with and was either maintained throughout the procedure or adjusted according to the audible “pops”. Postoperatively, an antibiotic/steroid ointment was instilled, and the eye patched over night. Antibiotic/steroid eye‐drops were prescribed 5 times daily for one week, and the preoperative glaucoma medication continued. Eyes were examined on the next day, at 1 and 4 weeks, and then according to the clinical picture. When a pressure‐lowering effect was noted, the hypotensive medication was reduced gradually, starting with oral acetazolamide. CPC was repeated (re‐treatment) if the IOP response was inadequate, after a minimum interval of 4 weeks.
Success was defined as IOP at last visit between 6 and 21 mm Hg, or an IOP of 22–26 mm Hg and pressure reduction of at least 30% compared with pretreatment, with or without topical medications. A decrease in the number of medications was also considered (eg, preop 20 mm Hg with 4 topically applied substances and a systemic carboanhydrase inhibitor, postop 17 mm Hg with two topical preparations only). Hypotony was considered when the IOP was ⩽5 mm Hg. The delivered laser energy (J) was calculated per pulse (pulse power (W) multiplied by pulse duration (s)), per treatment session (pulse energy multiplied by the number of laser burns) and per eye (all treatment sessions added).
To evaluate the outcome, the following parameters were calculated (as a percentage of the total): response rate (successful and hypotony eyes taken together), success rate, hypotony rate, re‐treatment rate (eyes with more than one treatment). Statistical analysis was performed with SPSS for Windows, version 13.0. Parameters were compared using the unpaired t test and Mann–Whitney test. The distribution of diagnosis between subgroups was compared with the Kruskal–Wallis test. Differences were considered significant when p<0.05. Data were reported as mean (SD), range minimum to maximum.
Results
During the 5 years considered in the study, a total of 181 eyes of 176 patients were treated with DCPC. A total of 268 treatment sessions were carried out, two‐thirds (67.5%) being initial cyclodiode treatments and one‐third (32.5%) re‐treatments. The number of treatments per year ranged between 42 and 65, mean 53.6. A mean of 36 new eyes (initial treatments) were registered per year.
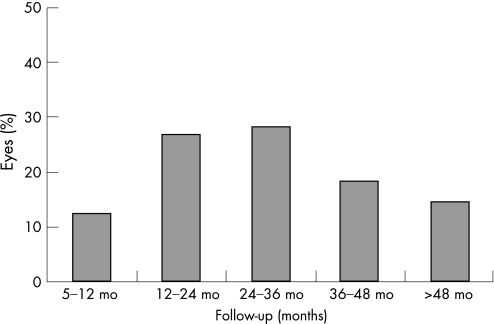
Fifty eyes had to be excluded because the documented follow‐up was less than 5 months. Thus, 131 eyes of 127 patients with a follow‐up of 5 or more months were included in the statistical analysis. There were 62 female and 65 male patients with mean age of 69.51 (SD 15.21), range 8–92 years, including only 7 patients under the age of 40. The mean follow‐up was 2.5 years or 30.1 (16.7) months (range 5–75 months). The distribution of the eyes according to the follow‐up period is given in fig 1. For these 131 eyes, 202 treatment sessions were carried out, a mean of 1.54 sessions per eye. Eighty‐nine per cent of the eyes had 1 or 2, and 11% had 3 or more treatments (table 1). The three subgroups—with 1 (n = 80), 2 (n = 37) and 3 or more (n = 14) cyclodiode treatments—were compared for age (Mann–Whitney test) and distribution of glaucoma diagnosis (Kruskal–Wallis test, adjusted for ties). There was no significant difference between the first two groups for any of the parameters. The patients in the third subgroup were significantly younger (p<0.001) and had significantly more post‐traumatic glaucomas (p<0.01) than the other two subgroups. The difference in the distribution of the other glaucoma types did not reach statistical significance.
Figure 1 Distribution of the study eyes (%) according to the follow‐up period.
Table 1 Distribution of eyes by number of treatment sessions and diagnosis.
| No. of treatment sessions | No. of eyes | Percentage (%) | Follow‐up, months (SD) | Age, years (SD) | NVG | Post‐VR | Trauma | ACG | POAG | XFG | PKP | Uveitis | Cong | Other sec gl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | 61.1 | 26.1 (15.1) | 72.8 (10.9) | 55 | 6 | – | 1 | 3 | 8 | 3 | 1 | 2 | 2 |
| 2 | 37 | 28.2 | 33.4 (14.7) | 68.1 (19.5) | 19 | 4 | 2 | – | 2 | 3 | – | 2 | – | 4 |
| 3 | 10 | 7.6 | 36.7 (20.6) | |||||||||||
| 4 | 3 | 2.3 | 64.0 (10.2) | 54.7 (15.5) | 6 | 3 | 4 | 1 | – | – | – | – | – | – |
| 6 | 1 | 0.8 | 60.3 | |||||||||||
| Total | 131 | 100 | 30.1 (16.7) | 69.5 (15.2) | 80 | 13 | 6 | 2 | 5 | 11 | 3 | 3 | 2 | 6 |
NVG, neovascular glaucoma; Post‐VR, glaucoma postvitreo‐retinal surgery; ACG, chronic angle‐closure glaucoma; Trauma, post‐traumatic glaucoma; POAG, primary open‐angle glaucoma; XFG, pseudo‐exfoliation glaucoma; PKP, glaucoma secondary to penetrating keratoplasty; Uveitis, uveitic glaucoma; Cong, congenital glaucoma; Other sec gl, secondary glaucomas of other aetiologies (untreated retinal detachment, complicated intraocular surgery, irido‐corneal‐endothelial syndrome).
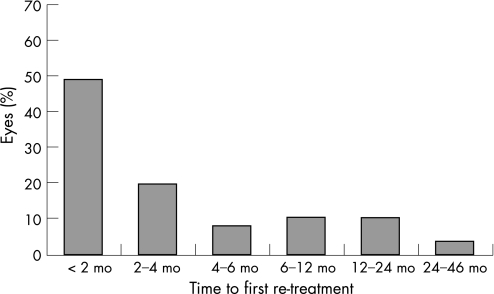
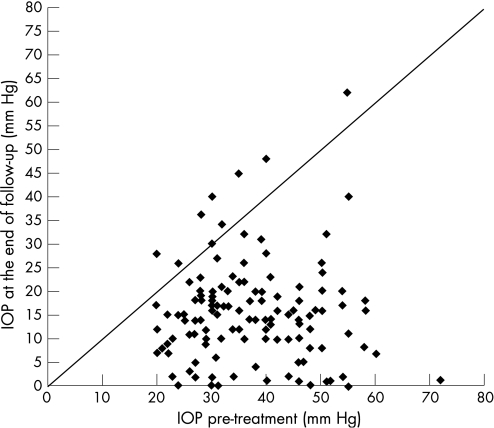
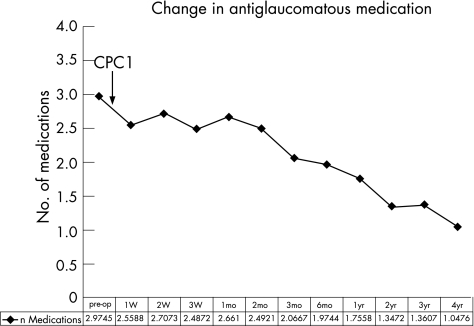
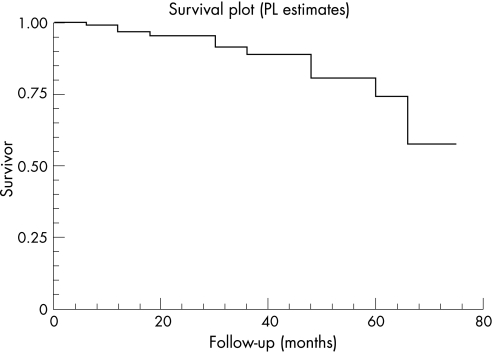
Fifty‐one eyes have received more than 1 cyclodiode session (re‐treatment rate 38.9%). The mean period between the initial and the second session (first re‐treatment) was 5.35 (SD 8.22) months (range 0.5 to 45.7 months). There were 5 eyes that were re‐treated before the completion of the first 4 weeks, because of a very high IOP. Almost 50% (25/51) of the re‐treatments occurred within the first 2 months, and almost 70% (35/51) within the first 4 months (fig 2). For the whole group, IOP decreased from 36.9 (10.7) mm Hg before initial DCPC to 15.3 (10.4) mm Hg at the end of follow‐up, a highly significant reduction of 55.5% (p<0.001) (fig 3). The mean number of antiglaucoma medications decreased significantly from 2.97 (1.36) pretreatment to 1.39 (1.36) at the end of follow‐up (p<0.001) (fig 4). Success was noted in 69.5% (91 eyes), failure (non‐response) in 13.0% (17 eyes) and hypotony in 17.6% (23 eyes, of which 13 were with phthisis). The overall response rate was 87%. Figure 5 presents the survival curves for the pressure response to DCPC treatment.
Figure 2 Time (in months) to the first cyclodiode re‐treatment.
Figure 3 Intraocular pressure (IOP) pre‐ and post‐treatment
Figure 4 Change in medication in the course of follow‐up.
Figure 5 Kaplan–Meier survival curves for the pressure response to diode laser cyclophotocoagulation, single and repeated treatments.
Mild uveitis within the first 2 weeks of treatment was observed frequently and was considered a common postoperative reaction rather than a complication. During the long‐term follow‐up in this study, by far the most frequent complication (17.6%; 23/131) was hypotony, 74% of these eyes having neovascular glaucoma. Hypotony occurred on a mean of 19.3 (SD 11.0) months after the initial treatment, range 6 to 36 months. Other complications included: prolonged uveitis 1, severe dry eye 1, hyphema 1, vitreous haemorrhage 1, keratitis/band keratopathy 4. It was not always clear‐cut whether the complication was related to the cyclodiode treatment itself or to the complexity of eye pathology (eg, corneal failure in an eye with several intraocular surgeries and periods of very high IOP with corneal oedema). No cases of endophthalmitis or sympathetic ophthalmia were observed.
When the whole study group and the entire follow‐up period were considered, 45.8% of the eyes (60/131) achieved a successful IOP control after the first cyclodiode treatment, and 9.2% (12/131) developed hypotony, that is 72 eyes responded to a single treatment. Following the second DCPC session, an additional 17.6% (23 eyes) gained control, but also another 7.6% (10 eyes) progressed to hypotony. Thus, after 1 or 2 treatment sessions, the IOP response rate was 80.2%, consisting of 63.4% with reasonable IOP control, and 16.8% with an “overshoot” leading to hypotony. A third cyclodiode session added another 7 eyes (5.3%) to the successful ones. There were 3 eyes with a total of 4 DCPC sessions—one gained control (IOP 10 mm Hg without medication), 1 developed hypotony (IOP 4 mm Hg), and 1 was non‐responsive. One traumatic eye has been treated 6 times with DCPC during 5 years, but the pressure was still 26 mm Hg.
The mean total energy delivered per eye was 133.9 (SD 73.7) J. When the energy delivered during the first session in the 72 responding eyes (92.5 (22.5) J) was compared with the energy in the 59 non‐responding eyes (77.2 (19.5) J), a significant difference was found (p<0.001). Also, 75% of the responding eyes have received a laser energy of 80.0 J and higher during the first DCPC, this proportion being 49% in the non‐responding eyes. However, the above relation was no longer observed after the second DCPC session: the total energy from the initial and the re‐treatment in the 33 responding eyes (166.7 (30.4) J) did not differ from that in the 18 non‐responding eyes (161.1 (27.3) J), p = 0.52. Interestingly, when the energy delivered during the first DCPC to the success eyes (n = 60) and to the hypotony eyes (n = 12) was compared, no difference was noted, p = 0.93. The same was true, when the successful and the hypotony eyes after one re‐treatment were compared, implicating that the occurrence of hypotony was not directly correlated with the laser energy.
Diagnosis distribution is given in table 2. The highest failure rate (the lowest response rate) was noted for eyes with traumatic glaucoma and pseudo‐exfoliation glaucoma (Kruskal–Wallis, adjusted for ties, p<0.01). It should be noted that 90% of XFG were very advanced cases with vision of hand motions to light perception. Traumatic glaucoma and glaucoma post‐VR surgery had a significantly higher number of treatments per eye compared with the other groups (p<0.05; unpaired t test and Mann–Whitney test). The differences between the other groups did not reach statistical significance. Hypotony was observed in NVG, glaucoma postvitreo‐retinal surgery, and secondary glaucoma of other aetiologies. Neovascular glaucoma was the largest diagnostic subgroup (61.1%). The subcategories of NVG are listed in table 3; no statistically significant difference was noted between these subcategories.
Table 2 Distribution of diagnosis.
| Diagnosis | No. of eyes | % of total | Response | Non‐response | No. of sessions per eye | Mean (SD) total energy (J) | Mean (SD) energy first treatment (J) | |
|---|---|---|---|---|---|---|---|---|
| Success | Hypotony | Failure | ||||||
| NVG | 80 | 61.1 | 56 | 17 | 7 | 1.4 | 123.9 (62.6) | 86.5 (20.1) |
| Post‐VR surgery | 13 | 9.9 | 10 | 3 | 1.9 | 158.8 (79.7) | 86.8 (20.1) | |
| Traumatic glaucoma | 6 | 4.6 | 3 | 3 | 3.2 | 273.4 (132.0) | 75.7 (12.9) | |
| Uveitic glaucoma | 3 | 2.3 | 3 | 1.7 | 117.0 (23.8) | 68.8 (20.7) | ||
| Postkeratoplasty | 3 | 2.3 | 2 | 1 | 1.0 | 83.3 (47.3) | 83.3 (47.3) | |
| Other secondary glaucomas | 6 | 4.6 | 2 | 3 | 1 | 1.6 | 131.2 (44.3) | 86.8 (20.1) |
| Congenital glaucoma | 2 | 1.5 | 2 | 1.0 | 110.0 (14.1) | 110.0 (14.4) | ||
| CACG | 2 | 1.5 | 2 | 2.0 | 250.0 (127.3) | 120.0 (56.6) | ||
| POAG | 5 | 3.8 | 5 | 1.4 | 135.0 (47.3) | 84.1 (33.4) | ||
| XFG | 11 | 8.4 | 6 | 5 | 1.3 | 103.7 (42.3) | 83.9 (19.8) | |
| Total | 131 | 100 | 91 | 23 | 17 | 1.54 | 133.9 (74.0) | 85.7 (22.5) |
Abbreviations as in table 1. All eyes with traumatic glaucoma had 2 or more CPC sessions (range 2–6); re‐treatment rate 100%. Congenital glaucoma: both eyes of the same patient with glaucoma fere absolutum, aphakia and high myopia. Chronic angle closure glaucoma: 2 eyes of 2 patients; the one with 3 filtering surgeries and penetrating keratoplasty because of bulous keratopathy; the other with microphthalmus, status postfiltering surgeries, cataract extraction; and trisomy 21.
Table 3 Composition of the subgroup of 80 eyes with neovascular glaucoma (NVG).
| Diagnosis | No. of eyes | % of total | Response | Non‐response | No. of sessions per eye | Mean (SD) total energy (J) | Mean (SD) energy first treatment (J) | |
|---|---|---|---|---|---|---|---|---|
| Success | Hypotony | Failure | ||||||
| NVG diabetes | 38 | 47.5 | 29 | 6 | 3 | 1.4 | 113.2 (46.1) | 84.3 (18.4) |
| NVG vein occlusion | 34 | 42.5 | 25 | 6 | 3 | 1.4 | 133.0 (75.8) | 90.9 (20.3) |
| NVG artery occlusion | 2 | 2.5 | 1 | 1 | 2.0 | 148.8 (29.4) | 61.8 (19.5) | |
| NVG other | 6 | 7.5 | 1 | 4 | 1 | 1.5 | 131.1 (78.8) | 88.2 (20.2) |
| NVG all | 80 | 100 | 56 | 17 | 7 | 1.4 | 123.9 (62.6) | 86.5 (20.1) |
Discussion
The results of this study confirm that refractory glaucoma can be managed on a long‐term basis (up to 6 years) with single or repeated cyclodiode treatments, the overall pressure‐lowering response rate being 87%, and the overall success rate approximately 70%. The outcome seems to be related to the type of glaucoma, the longevity of observation, the definition of success and, to a lesser extent, the laser parameters and delivered energy. Table 4 compares several larger studies published recently and allows some general conclusions: in high‐pressure glaucoma, an IOP decrease of 35–55% and a significant reduction in the number of medications can be expected. The response rate after one treatment and one re‐treatment is roughly 80%. This is particularly true for advanced, refractory cases. DCPC as primary interventional treatment and in primary glaucoma seems to be associated with less IOP decrease and less reduction of medication although, also, with fewer re‐treatments.18,19 In mixed cohorts, the proportion of neovascular glaucoma cases is relevant for the overall outcome. Comparing the four studies with intractable glaucomas (last four in table 4), it should be noted that Pucci et al17 used a very restrictive laser protocol with low power settings and, particularly, a low number of burns per treatment session. Although their reported overall success does not differ significantly from the other three studies, Pucci et al17 had the lowest percentage of IOP reduction, the highest postoperative IOP and the highest re‐treatment rate, this despite a very high proportion of primary glaucoma in their cohort. This may be due to the low‐energy protocol used.
Table 4 Comparison of 7 recent studies with mid‐ and long‐term follow‐up of eyes treated with diode‐laser cyclophotocoagulation.
| Study | Country | Follow‐up (months) | No. of eyes | Diagnosis | % Refractory glaucoma | % Neovasc glaucoma | % Primary glaucoma | Mean IOP pretreatm | Mean IOP end of FU | % IOP reduction | Overall success rate (%) | Definition of success | Re‐treatment rate (%) | Response rate after 1st CPC (%) | Response rate after 2nd CPC (%) | No. of treatments per eye | No. of treatments (range) | No. of burns per treatment episode | Mean energy per treatment episode (J) | Mean total energy per eye (J) | VA stable (%) | VA improved (%) | VA worsened (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kramp et al19 | Germany | 13.9 | 193 | Primary and secondary glaucoma | 6.2 | 64.8* | 24.6 | 19.3 | 21.5* | 76.4 | 10 to 22 mm Hg | 21.2 | 68.9 | 1.3 | 1 to 6 | 24 to 30 | 92.4* | 120.1* | |||||
| Grueb et al18 | Germany | 24.0 | 90 | Primary glaucoma | 0 | 100 | 21.0 | 16.0 | 23.8 | 36.7 | 4 to 18 mm Hg | 30.0 | 1.3 | 1 to 3 | 15 to 20 | 80.0 | 104.0* | ||||||
| Ansari and Gandhewar20 | UK | 12.5 | 74 | Refractory and non‐refractory glaucom | 50 | 31 | 40.3 | 21.1 | 45.1 | 82.0 | 30% reduction | 1.4† | 82** | 1.01 | 1 to 2 | 30 (4.5) | 124.1* | 124.1* | |||||
| Noureddin et al15 | Lebanon | 13.7 | 36 | Refractory glaucoma | 100 | 16.6 | 25 | 35.8 | 19.1 | 53 | 72.2 | ?? 21 mm Hg | 25.0 | 1.25 | 1 to 2 | 26 to 28 | 121.5* | 151.9* | 44 | 33 | 22 | ||
| Murphy et al16 | UK | 17.0 | 263 | Refractory glaucoma | 100 | 46.4 | 18.6 | 40.7 | 17.7 | 52.6 | 79.5 | 5 to 21 mm Hg | 34.2 | 58.9 | 81.3 | 1.5 | 1 to 7 | ‐ | 104.1 | 155.2 | 75 | 5 | 20 |
| Pucci et al17 | Italy | 26.0 | 120 | Refractory glaucoma | 100 | 12.5 | 52.5 | 30.4 | 20.3 | 35 | 76‡ | ?? 21 mm Hg | 45.8 | 54.1 | 1.7 | 1 to 5 | 10 (1.8) | 43.6* | 75.2* | 55 | 21 | 24 | |
| Iliev and Gerber (this study) | Switzerland | 30.1 | 131 | Refractory glaucoma | 100 | 61.1 | 12.2 | 37.7 | 15.3 | 55 | 69.5 | 6 to 21 mm Hg | 38.9 | 55.0 | 80.2 | 1.54 | 1 to 6 | 22 (5.6) | 86.8 | 133.9 | 55 | 11 | 34 |
*Calculated, based on the data in the paper; †only one eye was re‐treated; ‡calculated according to table 2 in the paper; **see the definition of success and the end IOP.
Hypotony was the most common long‐term complication in our study (17.6%). This complication was observed only in 3 diagnostic categories, neovascular glaucoma, glaucoma post vitreo‐retinal surgery and secondary glaucoma of other aetiologies (table 2), which is in accordance with other published reports.16 Studies with cohorts comprising different diagnostic categories, mainly primary glaucomas, have reported significantly lower hypotony rates.17,18,19 Murphy et al16 noted a hypotony rate of 15.6% in a large subgroup of 122 eyes with NVG. Their definition of hypotony was “less than 5 mm Hg”. If we adjust our criterion accordingly, the hypotony rate in our NVG subgroup becomes 17.5% (14/80), which is comparable with that reported by Murphy et al.16
We analysed various parameters for association with hypotony. However, hypotony incidence did not depend upon the laser energy delivered during initial DCPC treatment, and did not increase (ie, remained stable) after the second treatment. Eyes developing hypotony have not received higher energies or more re‐treatments compared with eyes maintaining normal IOP. Half of the eyes complicating to hypotony (12 of 23) have had only 1 DCPC session, the other 10 eyes 2, and only 1 eye had 4 sessions. Hypotony occurred on a mean of 19.3 months after the initial treatment, as early as 6 months and as late as 36 months. The mean age was not significantly different between the subgroups with hypotony, successful pressure control and pressure non‐response. In conclusion, an overshoot reaction to cyclodiode and development of hypotony cannot be predicted in an individual case but should be expected in 15–20% in neovascular glaucoma, and in an even higher percentage in glaucoma postvitreoretinal or other intraocular surgeries. A longer follow‐up is likely to be associated with new cases of hypotony and phthisis. The intention to reduce hypotony risk by using a lower laser power and fewer applications per treatment should be weighed against the possible lower response rate.
The overall re‐treatment rate (all eyes with more than a single DCPC) was 39%, younger patients, post‐traumatic cases, and glaucoma post‐VR surgery tending to need higher number of repetitions. Although the bulk of first re‐treatments took place within 4 months of initial DCPC, more and more initially successful eyes had to be re‐treated over time. Regardless of this trend, it does not appear possible to define an “average duration of cyclodiode success”, since more than 50% of the eyes controlled with a single treatment had a follow‐up over 2 years (ie, significantly longer than the average time to re‐treatment) and 25% over 3 years, with the longest follow‐up of a successful IOP control after a single treatment being 5.5 years in this study cohort.
In conclusion, diode cyclophotocoagulation is an efficient treatment for refractory glaucoma. Diagnostic category and age seem to influence postoperative course and outcome stronger than laser protocol and delivered energy. Neovascular glaucoma and glaucoma secondary to intraocular surgery are associated with a higher incidence of hypotony, while post‐traumatic, pseudo‐exfoliation glaucoma and younger age tend to be less responsive to cyclodiode treatment.
Abbreviations
DCPC - diode‐laser cyclophotocoagulation
FU - follow‐up
IOP - intraocular pressure
NVG - neovascular glaucoma
Footnotes
Financial interest: None.
Competing interest: None.
References
- 1.Nicaeus T, Derse M, Schlote T.et al Cyclocryocoagulation in treatment of therapy refractory glaucoma: a retrospective analysis of 185 cryocoagulation procedures. Klin Monatsbl Augenheilkd 1999214224–230. [DOI] [PubMed] [Google Scholar]
- 2.Shields M B, Shields S E. Noncontact transscleral Nd:YAG cyclophotocoagulation: a long‐term follow‐up of 500 patients. Trans Am Ophthalmol Soc 199492271–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Fankhauser F, Kwasniewska S. The role of laser cyclocoagulation in cyclodestructive glaucoma surgery. Curr Opin Ophthalmol 1993479–84 Review. [PubMed] [Google Scholar]
- 4.Kosoko O, Gaasterland D E, Pollack I P.et al Long‐term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 19961031294–1302. [DOI] [PubMed] [Google Scholar]
- 5.Brancato R, Carassa R G, Bettin P.et al Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma. Eur J Ophthalmol 1995532–39. [DOI] [PubMed] [Google Scholar]
- 6.Assia E I, Hennis H L, Stewart W C.et al A comparison of neodymium: yttrium aluminum garnet and diode laser transscleral cyclophotocoagulation and cyclocryotherapy. Invest Ophthalmol Vis Sci 1991322774–2778. [PubMed] [Google Scholar]
- 7.Ulbig M W, McHugh D A, McNaught A I.et al Clinical comparison of semiconductor diode versus neodymium: YAG non‐contact cyclophotocoagulation. Br J Ophthalmol 199579569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn J, Cox T A, Herndon L W.et al A clinical comparison of transscleral cyclophotocoagulation with neodymium: YAG and semiconductor diode lasers. Am J Ophthalmol 1998126640–647. [DOI] [PubMed] [Google Scholar]
- 9.Bloom P A, Tsai J C, Sharma K.et al Cyclodiode: trans‐scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 19971041508–1519. [DOI] [PubMed] [Google Scholar]
- 10.Mistlberger A, Liebmann J M, Tschiderer H.et al Diode laser transscleral cyclophotocoagulation for refractory glaucoma. J Glaucoma 200110288–293. [DOI] [PubMed] [Google Scholar]
- 11.Schlote T, Derse M, Zierhut M. Transscleral diode laser cyclophotocoagulation for the treatment of refractory glaucoma secondary to inflammatory eye diseases. Br J Ophthalmol 200084999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlote T, Derse M, Rassmann K.et al Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma 200110294–301. [DOI] [PubMed] [Google Scholar]
- 13.Walland M J. Diode laser cyclophotocoagulation: longer term follow up of a standardized treatment protocol. Clin Exp Ophthalmol 200028263–267. [DOI] [PubMed] [Google Scholar]
- 14.Chang S H, Chen Y C, Li C Y.et al Contact diode laser transscleral cyclophotocoagulation for refractory glaucoma: comparison of two treatment protocols. Can J Ophthalmol 200439511–516. [DOI] [PubMed] [Google Scholar]
- 15.Noureddin B N, Zein W, Haddad C.et al Diode laser transcleral cyclophotocoagulation for refractory glaucoma: a 1 year follow‐up of patients treated using an aggressive protocol. Eye 200620329–335. [DOI] [PubMed] [Google Scholar]
- 16.Murphy C C, Burnett C A, Spry P G.et al A two centre study of the dose–response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol 2003871252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pucci V, Tappainer F, Borin S.et al Long‐term follow‐up after transscleral diode laser photocoagulation in refractory glaucoma. Ophthalmologica 2003217279–283. [DOI] [PubMed] [Google Scholar]
- 18.Grueb M, Rohrbach J M, Bartz‐Schmidt K U.et al Transscleral diode laser cyclophotocoagulation as primary and secondary surgical treatment in primary open‐angle and pseudoexfoliatve glaucoma: Long‐term clinical outcomes. Graefes Arch Clin Exp Ophthalmol 20062441293–1299. [DOI] [PubMed] [Google Scholar]
- 19.Kramp K, Vick H P, Guthoff R. Transscleral diode laser contact cyclophotocoagulation in the treatment of different glaucomas, also as primary surgery. Graefes Arch Clin Exp Ophthalmol 2002240698–703. [DOI] [PubMed] [Google Scholar]
- 20.Ansari E, Gandhewar J. Long‐term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non‐refractory glaucoma. Eye. 2006. Published Online First 21 April 2006. doi: 10.1038/sj.eye.6702345 [DOI] [PubMed]