Abstract
Hepatitis C virus (HCV) is well known for its aetiological role in chronic non‐A, non‐B viral hepatitis, liver cirrhosis and hepatocellular carcinoma; in addition, the virus has also been implicated in a number of extra‐hepatic “autoimmune” disease manifestations. A causative association between HCV and non‐Hodgkin lymphoma (NHL) was postulated relatively recently and has been the subject of intense investigation, as well as some debate. On the strength of epidemiological data, emerging biological investigations and clinical observations, HCV appears to be involved in the pathogenesis of at least a proportion of patients with NHL. Morphologically, HCV‐associated lymphomas represent a variety of histological subtypes including marginal zone lymphoma (splenic, nodal and extranodal), small lymphocytic lymphoma/chronic lymphocytic leukaemia, lymphoplasmacytic lymphoma and diffuse large B‐cell lymphoma. Remarkably, some HCV‐associated NHL appears to be highly responsive to antiviral therapy, providing some clinical evidence for this relationship, as well as the prospect for novel therapeutic intervention.
The relationship between lymphoproliferative disorders and infectious agents has been recognised and studied for many decades. Some viral or microbial pathogens, such as Epstein–Barr virus (EBV) and Helicobacter pylori have relatively well documented aetiological roles in the pathogenesis of lymphoma, whereas other common agents (eg HIV) serve to create the pathobiological milieu conducive to lymphomagenesis. Hepatitis C virus (HCV) is a small (∼9600 nucleotide) encapsulated positive strand RNA member of the Flaviviridae family. The virus lacks a reverse‐transcriptase and its genome encodes a single open reading frame for a large polyprotein, which is subsequently cleaved to several structural and non‐structural (enzymatic) component viral proteins. Antibodies generated in response to viral proteins can be detected by serological methods and the presence of the virus subsequently confirmed directly by reverse‐transcriptase (RT)‐PCR or related molecular assays in the clinical laboratory. Because of genetic instability generated during viral replication, several major genotypes of HCV with differing global distribution are recognised1,2,3,4 and can be determined by molecular typing. Certain genotypes of the virus have been associated with poor response to interferon or other antiviral therapies. HCV is well known for its aetiological role in chronic non‐A, non‐B viral hepatitis, liver cirrhosis and hepatocellular carcinoma; in addition, the virus has also been implicated in a number of extra‐hepatic “autoimmune” disease manifestations. A causative association between HCV and non‐Hodgkin lymphoma (NHL) was postulated relatively recently and has been the subject both of intense investigation and of some debate. On the strength of epidemiological data, emerging biological investigations and clinical observations, HCV appears to be involved in the pathogenesis of at least a proportion of patients with NHL. This review will summarise current knowledge concerning HCV and its possible role in the production of lymphoma in susceptible individuals.
HCV and mixed cryoglobulinaemia
Acute HCV infection induces both humoral and cell‐mediated immune responses, although these reactions are apparently insufficient to prevent the development of chronic infection and persistent viraemia in the majority of patients. In the setting of this chronic antigenic stimulation, a variety of localised or systemic autoimmune disorders (eg Sjögren syndrome, polyarteritis nodosa) can potentially develop in HCV positive individuals.5,6 One manifestation of autoimmune dysregulation of normal B‐cell physiology is the phenomenon of cryoglobulinaemia. Cryoglobulins are immune globulins that by definition precipitate in serum incubated at 4°C. Insolubility is determined in part by serum cryoglobulin concentration, leading to pathological effects frequently occurring at more ambient temperatures in many patients. Cryoglobulins are classified into three major types, based on the presence or absence of a monoclonal immunoglobulin. Type I proteins are characteristic of lymphoplasmacytic lymphoma and the associated clinical syndrome of Waldenström's macroglobulinaemia; accordingly, type I cryoglobulinaemia is associated with a single monoclonal IgM paraprotein. Type II disease, also referred to as mixed cryoglobulinaemia (MC), results from the production of a monoclonal rheumatoid factor‐like IgM autoantibody acting against polyclonal IgG class immunoglobulins. Circulating immune complexes produce the clinical manifestations of inflammatory disease, including arthritis and vasculitis. Type II MC can be identified in many patients with rheumatoid arthritis. A second sub‐class of mixed cryoglobulinaemia (type III) is characterised by polyclonal IgM–anti‐IgG immunoglobulins and is also seen in association with inflammatory disorders or chronic infections. Intriguingly, a high percentage of patients with MC are HCV positive. Conversely, the prevalence of type II (and less frequently type III) MC occurring among HCV positive individuals appears more variable in published studies, although some geographic regions of the world (eg Italy) have quite high rates, approaching 40–50% of patients; these widespread disparities reflect not only differences in patient populations, but also other factors, such as laboratory analytic methodology (summarised in Mazzaro et al7). While cryoglobulins are frequently detected in HCV positive individuals, most cases are relatively asymptomatic and only a minority of individuals (5–10%) develop overt vasculitic disease.6
The basis for MC in the setting of HCV infection is not precisely clear; however, analysis of monoclonal IgM antibodies from different individuals reveals similar idiotype specificities, often favouring use of the IgH‐V1‐69 and IgK‐V3‐20 immunoglobulin segments (summarised in Libra et al8). These studies indicate the propensity of HCV to induce a sustained, restricted B‐cell repertoire in response to chronic antigenic stimulation. Indeed, molecular clonality investigations of hepatic, bone marrow or peripheral blood lymphocytes have revealed the presence of monoclonal B‐cell populations in these patients.9,10,11,12,13 By extension, MC occurring in the setting of HCV could be considered a form fruste of a chronic B‐cell lymphoproliferative disorder,14 and long term studies indeed suggest that nearly 10% of patients may develop overt NHL.15 In keeping with these observations, HCV patients treated with interferon‐alpha have been reported to show a regression in MC and MC‐related clonotypic peripheral blood B‐cells.16,17,18,19
Epidemiology and histopathology of HCV‐associated NHL
Evidence linking HCV to NHL derives from a large number of epidemiological studies, which have nonetheless provided variable data reflecting differences in study designs, patient populations, geographic regions, HCV prevalence and laboratory methods. Despite many such inconsistencies, both recent and older reports generally support a relationship between chronic HCV infection and an elevated risk for developing NHL. HCV positive individuals from the Mediterranean region (especially Southern Italy), Japan, Brazil and possibly Eastern Europe, show a modestly increased overall risk association (odds ratios 2–4) with NHL (summarised in Negri et al20). In contrast, Northern European, UK and Canadian patient populations appear to have no convincing epidemiological evidence to support a link between HCV and NHL. Many studies showing a significant association with lymphoma were notably performed in geographic regions with highest HCV prevalence. In the USA, earlier epidemiological investigations revealed rather disparate findings, with an increased prevalence of HCV positivity among NHL cases in New Orleans and Los Angeles study populations (summarised in Negri et al20), but no association in a study from Southern Florida state.21 A report involving women from the state of Connecticut suggested a trend towards increased risk.22 These US studies raised the issue of having insufficient patient numbers in order to demonstrate and quantify lymphoma risk with sufficient statistical power in lower HCV prevalence areas. To this end, two recent large US studies involving the NCI‐SEER registry and the US Veterans Affairs health system respectively, as well as an analysis by the European multicentre EPILYMPH consortium, have documented a positive, modestly increased risk of NHL in patients with HCV as compared to HCV‐negative controls (relative risk (RR) = 2–3).23,24,25 These latter studies are additionally supported by a concurrent meta‐analysis review of 15 case‐control and 3 prospective studies in this field, showing a “pooled” RR of 2–2.5 depending on study design.26 From the aggregate epidemiological data, it thus appears that there is a greater propensity to develop NHL in the setting of HCV, that the risk is most dramatically evident in populations with high HCV prevalence, and that geographic variability worldwide may indicate additional important environmental factors influencing the strength of this relationship. While an association with particular HCV genotypes has been suggested by some groups,25 this aspect remains controversial.
Morphologically, HCV‐associated lymphomas represent a variety of WHO types, including marginal zone (splenic, nodal and extranodal) (figs 1 and 2), small lymphocytic lymphoma/chronic lymphocytic leukaemia, lymphoplasmacytic lymphoma (fig 3) and diffuse large B‐cell lymphoma (DLBCL). No pathological features that could distinguish NHL arising in a background of HCV from sporadic cases have been described so far. The sub‐classification of HCV‐associated low grade lymphomas has been somewhat problematic given the use of confusing and overlapping nomenclature in the literature, such as lymphoplasmacytoid lymphoma and immunocytoma. Some epidemiological studies have indicated a stronger relationship of specific lymphoma types with HCV infection, including diffuse large B‐cell, marginal zone B‐cell and lymphoplasmacytic,25 whereas other investigators have reported consistently increased risk association among most B‐cell lymphoma types and even T‐cell lymphomas.26,27 Overall, marginal zone lymphoma appears to be the most frequently encountered low grade B‐cell lymphoma in HCV patients. Concerning large B‐cell lymphomas in HCV positive patients, some investigators have suggested that the majority of these tumours arise from underlying low grade B‐cell lymphomas, likely of marginal zone type. HCV positive patients with DLBCL have an inferior prognosis compared to HCV negative DLBCL patients, a finding attributed to the co‐morbidity of liver compromise by viral hepatitis.28
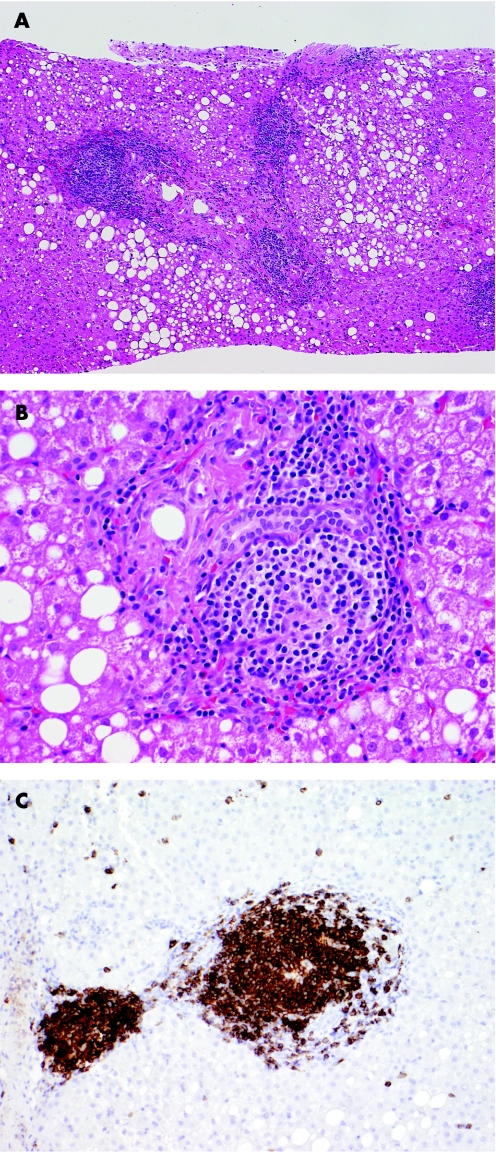
Figure 1 Histological appearances of a B‐cell lymphoma, best classified as a marginal zone lymphoma involving the liver. (A) Low power view of liver core biopsy shows chronic hepatitis, steatosis and marked portal lymphoid infiltrates. (B) High power view of liver core biopsy shows a monotonous portal lymphoid infiltrate composed of small lymphocytes with moderate amount of clear cytoplasm (so‐called monocytoid appearance). Note that the infiltrate does not involve the biliary epithelium. (C) CD20 immunostain shows that virtually all of the lymphoid cells are B cells.
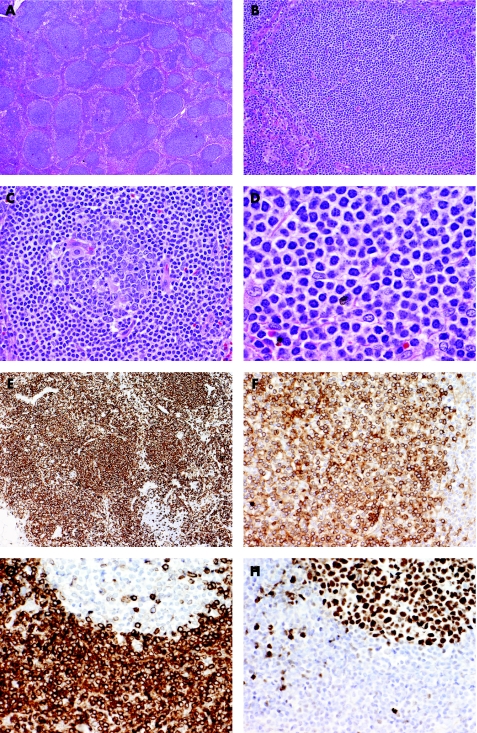
Figure 2 Nodal marginal zone lymphoma arising in a patient with chronic HCV hepatitis. (A) Although there is partial preservation of the lymph node architecture, there are numerous nodules/follicles primarily composed of small lymphoid cells. (B&C) Some of the follicles are entirely composed of small lymphoid cells mimicking primary follicles (B), but others contain a small reactive germinal centre and neoplastic cells occupy the mantle and marginal zones (C). (D) High power view shows that the neoplastic cells are mostly small lymphocytes with moderate amount of clear cytoplasm (so‐called monocytoid appearance). (E&F) Immunohistochemistry shows that vast majority of the neoplastic cells within the follicles but also in the interfollicular areas are CD20 B cells (E) which express immunoglobulin kappa light chain (F). (G&H) The neoplastic B cells have the typical phenotype of marginal zone B cells expressing BCL2 (G) but not BCl6 (H). Note that residual follicles express BCL6 but not BCL2.
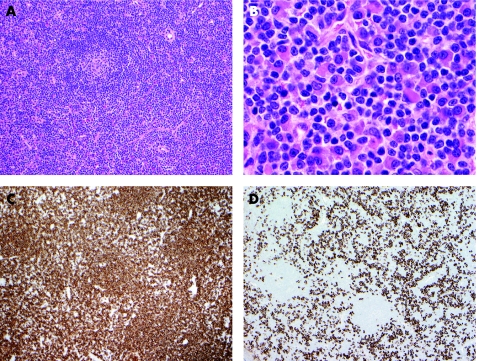
Figure 3 Lymphoplasmacytic lymphoma arising in a patient with chronic HCV hepatitis. (A) Low power view of the lymph node biopsy shows typical histology of lymphoplasmacytic lymphoma. There are occasional atrophic residual follicles but the most striking feature is the paracortical polymorphic neoplastic infiltrate. (B) High power view shows that the neoplastic cells are a mixture of small lymphocytes, lymphoplasmacytic cells and plasma cells. (C&D) Immunohistochemistry confirms lymphoplasmacytic nature of the infiltrate. Most of the small lymphocytes are CD20‐positive B‐cells (C) and these are intermixed with numerous CD138‐positive plasma cells (D).
Pathogenesis of HCV‐associated NHL
Although epidemiological data links HCV infection and NHL, the pathobiological processes leading to clonal B‐cell expansion and subsequent malignant transformation are only recently becoming better understood. Lacking a reverse transcriptase, the positive strand viral RNA molecule requires both viral and native intracellular proteins to generate a negative strand replicative intermediate. Thus, identification of negative coding strand viral RNA sequences in host cells is indicative of active virus replication. Negative strand intermediates are, not surprisingly, readily detected in hepatocytes, but the presence of active viral replication within haematopoietic cells has been a controversial matter (summarised in Weng and Levy29). Recently, one group of investigators, using highly purified mRNA preparations and a discriminating RT‐PCR technique, was able to show the presence of the negative strand HCV intermediate in the peripheral blood (13%) and bone marrow (11%) of 42 chronically infected patients.30 Mixed cryoglobulinaemia was exclusively found in 46% of the patients exhibiting active viral replication in blood or bone marrow cells, two of whom also had NHL. Despite these intriguing observations, a direct oncogenic role for HCV in B‐cell lymphomagenesis was not supported in this study given the absence of molecular evidence for active viral replication in the remaining chronically infected patients with either MC or NHL. Accordingly, the actual presence of replicating virions within some B‐cells may not be sufficient for the production of virus‐driven B‐cell expansion. Attention has therefore focused on selective viral interactions and effects of chronic HCV antigenic exposure on host B‐cell populations.
B‐cells are activated through engagement of the surface immunoglobulin/B‐cell antigen receptor complex (BCR), but can also be stimulated via a multi‐protein cell surface complex comprising the CD81 receptor, the signal transducer CD19 and the CD21 molecule.7,8,29 Significantly, synchronous co‐stimulation of the CD19/CD21/CD81 complex along with the BCR lowers the threshold for B‐cell activation and subsequent B‐cell proliferation.31 In this regard, CD81 has emerged as a potentially key mediator of B‐cell/HCV interaction, in light of the finding that CD81 can bind to at least two sites on the HCV envelope protein, E2. CD81/E2 interaction does not apparently promote viral entry into B‐cells32; however, B cells with specific anti‐HCV surface immunoglobulins can simultaneously interact with viral E2 protein via CD81, resulting in dual activation signals leading to B‐cell proliferation. This concept has been illustrated in vitro using anti‐CD81 antibodies to mimic the activation of B cells induced by HCV exposure33; most interestingly, the engagement of CD81 resulted in a polyclonal expansion of naïve (CD27 negative) B cells. Consequently, chronic antigenic stimulation may play a significant role in the development of an initial polyclonal B‐cell expansion, which may progress to autonomous B‐cell proliferation, immune dysregulation, and eventually B‐cell malignancy. The presence of recurrent clonotypic immunoglobulin idiotypes in B cells from different HCV positive patients with mixed cryoglobulinaemia supports this scenario. Furthermore, clonal immunoglobulin gene rearrangements from HCV‐positive lymphomas often share a similar restricted gene segment usage pattern as seen in B cells from patients with MC and show somatic hypermutation, emphasising the link between chronic viral antigenic stimulation and NHL pathogenesis.34,35
Recent investigations by Machida and colleagues36,37 have suggested a direct relationship between HCV infection and induction of acquired point mutations in both immunoglobulin and non‐immunoglobulin genes. Exposure of B‐cell lines and peripheral B cells to HCV in vitro was associated with a 5–10 fold increase in nucleotide mutations involving the immunoglobulin heavy chain gene, as well as several oncogenic targets (eg TP53, BCL6, β‐catenin). This increased mutation burden was similarly noted in HCV‐associated lymphomas and hepatocellular carcinomas (HCC), but was not detected in HCV‐negative lymphomas or hepatitis B virus‐associated HCC. Further investigations by this group identified induction of the nitric oxide pathway as a mechanism for generating point mutations in TP53 and other genes, but not the IgH locus. These authors subsequently demonstrated that HCV E2/CD81 receptor interaction induces DNA double strand breaks and up‐regulation of activation‐induced cytidine deaminase (AID), leading to IgH gene somatic hypermutation in the affected B cells.37 These studies point to a “mutator phenotype” role of HCV affecting a variety of genetic targets through different metabolic cellular pathways. Moreover, the presence of IgH locus somatic hypermutations in HCV exposed B cells may not necessarily reflect a “fine tuning” of adaptive B‐cell humoral immunity, but rather may represent an aberrant viral effect promoting pathological double strand DNA breakage and nucleotide substitution. As such, this situation may paradoxically produce an attenuation of epitope responsiveness by anti‐HCV‐specific B cells, allowing infection to persist and a susceptible B‐cell repertoire to become more extensively mutated, clonally selected and perhaps malignantly transformed. Other investigators, however, have not identified the somatic hypermutation process to be a key factor underlying non‐immunoglobulin gene alterations in MC B cells or HCV‐positive lymphomas in vivo, and indeed have shown that such mutations are actually present at lower frequency than in HCV negative lymphomas.38,39 Further investigations in this area are thus clearly warranted.
Additional deleterious genetic events are likely required to convert a clonal B‐cell expansion to a fully malignant lymphomatous process in HCV patients, analogous to the postulated molecular pathogenesis of H pylori‐induced gastric marginal zone lymphomas. While comparatively little is currently known about key genetic abnormalities in HCV‐associated lymphomas, it is of interest that B lymphocytes from HCV patients with MC show a high frequency of the BCL2‐IgH gene fusion, arising from the t[14;18](q21;q32) abnormality.40,41,42,43 Although the presence of this genetic abnormality is infrequently detected in HCV positive lymphomas, BCL2 gene rearrangements were identified more often in HCV‐positive than HCV‐negative patients with marginal zone type lymphomas.44,45
Clinical studies: response of HCV‐associated lymphoma to interferon therapy
The accumulating epidemiological and molecular biological data supporting the case for virally‐induced lymphomagenesis in some individuals with HCV infection are further reinforced by observations from small clinical studies demonstrating lymphoma regression in response to anti‐HCV therapy. The largest reported series of patients involved 18 individuals with primary splenic marginal zone lymphoma (splenic lymphoma with villous lymphocytes), the majority of whom (72%) had MC.46,47 Strikingly, 78% of patients responded completely to antiviral treatment (interferon +/− ribavirin) and achieved sustained haematological and clinical responses, including relief from symptoms associated with MC. Even patients with partial responses demonstrated varying degrees of haematological improvement proportional to the degree of reduction in viral load. One individual suffered a relapse of splenic lymphoma with the concomitant return of serum HCV RNA, then attained a second complete response upon reinstitution of antiviral therapy. Notably, patients who were tested for the presence of clonotypic immunoglobulin gene rearrangements by PCR technique all showed positive (monoclonal) results in blood samples, suggesting the presence of minimal residual lymphoma, delayed clearance of clonal neoplastic cells, or perhaps residual clonal, but non‐malignant, B cells. Although limited to small numbers of patients with primary splenic lymphomas, this antiviral therapeutic approach may be effective in other cases of HCV lymphoma, as suggested by the regression of clonotypic B‐cell populations in patients with MC.
Take‐home messages
Hepatitis C virus (HCV) has been increasingly linked to the development of non‐Hodgkin lymphoma on the strength of epidemiological investigations, biological studies and clinical and therapeutic observations.
The pathogenesis of HCV‐associated lymphoma involves chronic antigen stimulation of B‐cell populations, likely through enhanced engagement of B‐cell surface receptors and co‐stimulatory molecules.
A subset of patients with splenic marginal B‐cell lymphomas shows dramatic lymphoma regression on treatment with antiviral agents targeting HCV.
Conclusions
The field of investigation in HCV‐associated lymphoma has expanded from initial epidemiological associations to current molecular biological studies of pathogenesis, together providing increasingly convincing data for an aetiological relationship. It is apparent that the phenomenon of HCV lymphomagenesis is encountered most frequently in regions with relatively high HCV infection prevalence; aggregate epidemiological data reveal a modestly increased risk association overall. The mechanistic effects of viral/host B‐cell interaction have become better understood, indicating a role for chronic antigenic stimulation in the selection and expansion of B‐cell clones, establishing the background on which lymphoma can develop. As a consequence of HCV interactions with B‐cell surface receptors, aberrant somatic hypermutation affecting the immunoglobulin genes may occur and contribute to the propagation of a clonotypic B‐cell population, although the production of substantial mutagenic effects on non‐immunoglobulin gene targets remains controversial at present. In this regard, the latter observations suggest that HCV‐associated NHL share some of the pathogenic features of other recognised and potentially reversible “antigen‐driven” lymphoma syndromes, such as H pylori‐related gastric marginal zone B‐cell lymphoma.
Footnotes
Competing interests: None declared.
References
- 1.Simmonds P, Holmes E C, Cha T A.et al Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS‐5 region. J Gen Virol 1993742391–2399. [DOI] [PubMed] [Google Scholar]
- 2.Takada N, Takase S, Takada A.et al Differences in the hepatitis C virus genotypes in different countries. J Hepatol 199317277–283. [DOI] [PubMed] [Google Scholar]
- 3.Dusheiko G, Schmilovitz‐Weiss H, Brown D.et al Hepatitis C virus genotypes: an investigation of type‐specific differences in geographic origin and disease. Hepatology 19941913–18. [PubMed] [Google Scholar]
- 4.Mahaney K, Tedeschi V, Maertens G.et al Genotypic analysis of hepatitis C virus in American patients. Hepatology 1994201405–1411. [DOI] [PubMed] [Google Scholar]
- 5.Sterling R K, Bralow S P. Extrahepatic manifestations of hepatitis C virus. Curr Gastroenterol Rep 2006853–59. [DOI] [PubMed] [Google Scholar]
- 6.Zignego A L, Ferri C, Pileri S A.et al Extrahepatic manifestations of hepatitis C virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis 2007392–17. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaro C, Tirelli U, Pozzato G. Hepatitis C virus and non‐Hodgkin's lymphoma 10 years later. Dig Liver Dis 200537219–226. [DOI] [PubMed] [Google Scholar]
- 8.Libra M, Gasparotto D, Gloghini A.et al Hepatitis C virus (HCV) infection and lymphoproliferative disorders. Front Biosci 2005102460–2471. [DOI] [PubMed] [Google Scholar]
- 9.Franzin F, Efremov D G, Pozzato G.et al Clonal B‐cell expansions in peripheral blood of HCV‐infections patients. Br J Haematol 199590548–552. [DOI] [PubMed] [Google Scholar]
- 10.Sansonno D, De Vita S, Iacobelli A R.et al Clonal analysis of intrahepatic B cells from HCV‐infected patients with and without mixed cryoglobulinemia. J Immunol 19981603594–3601. [PubMed] [Google Scholar]
- 11.Magalini A R, Facchetti F, Salvi L.et al Clonality of B‐cells in portal lymphoid infiltrates of HCV‐infected livers. J Pathol 199818586–90. [DOI] [PubMed] [Google Scholar]
- 12.Racanelli V, Sansonno D, Piccoli C.et al Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus‐infected patients. J Immunol 200116721–29. [DOI] [PubMed] [Google Scholar]
- 13.Rasul I, Shepherd F A, Kamel‐Reid S.et al Detection of occult low‐grade B‐cell non‐Hodgkin's lymphoma in patients with chronic hepatitis C infection and mixed cryoglobulinemia. Hepatology 199929543–547. [DOI] [PubMed] [Google Scholar]
- 14.Saadoun D, Landau D A, Calabrese L H.et al Hepatitis C‐associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology 2007461234–1242. [DOI] [PubMed] [Google Scholar]
- 15.Ferri C, Sebastiani M, Giuggioli D.et al Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 200433355–374. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaro C, Franzin F, Tulissi P.et al Regression of monoclonal B‐cell expansion in patients affected by mixed cryoglobulinemia responsive to alpha‐interferon therapy. Cancer 1996772604–2613. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaro C, Pazzato G, Moretti M.et al Long‐term effects of alpha‐interferon therapy for type II mixed cryoglobulinemia. Haematologica 199479342–349. [PubMed] [Google Scholar]
- 18.Misiani R, Bellavita P, Fenili D.et al Interferon alpha‐2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med 1994330751–756. [DOI] [PubMed] [Google Scholar]
- 19.Casato M, Mecucci C, Agnello V.et al Regression of lymphoproliferative disorder after treatment for hepatitis C virus infection in a patient with partial trisomy 3, Bcl‐2 overexpression, and type II cryoglobulinemia. Blood 2002992259–2261. [DOI] [PubMed] [Google Scholar]
- 20.Negri E, Little D, Boiocchi M.et al B‐cell non‐Hodgkin's lymphoma and hepatitis C virus infection: a systematic review. Int J Cancer 20041111–8. [DOI] [PubMed] [Google Scholar]
- 21.Morgensztern D, Rosado M F, Silva O.et al Prevalence of hepatitis C infection in patients with non‐Hodgkin's lymphoma in south Florida and review of the literature. Leuk Lymphoma 2004452459–2464. [DOI] [PubMed] [Google Scholar]
- 22.McOmber Morten L, Engels E A, Holford T R.et al Hepatitis C virus and risk of non‐Hodgkin lymphoma: a population‐based case‐control study among Connecticut women. Cancer Epidemiol Biomarkers Prev 200413425–430. [PubMed] [Google Scholar]
- 23.Engels E A, Chatterjee N, Cerhan J R.et al Hepatitis C virus infection and non‐Hodgkin lymphoma: results of the NCI‐SEER multi‐center case‐control study. Int J Cancer 200411176–80. [DOI] [PubMed] [Google Scholar]
- 24.Giordano T P, Henderson L, Landgren O.et al Risk of non‐Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 20072972010–2017. [DOI] [PubMed] [Google Scholar]
- 25.Nieters A, Kallinowski B, Brennan P.et al Hepatitis C and risk of lymphoma: results of the European Multicenter Case‐Control Study EPILYMPH. Gastroenterology 20061311879–1886. [DOI] [PubMed] [Google Scholar]
- 26.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta‐analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2006152078–2085. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo K, Kusano A, Sugumar A.et al Effect of hepatitis C virus infection on the risk of non‐Hodgkin's lymphoma: a meta‐analysis of epidemiological studies. Cancer Sci 200495745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson C, Canioni D, Lepage E.et al Characteristics and outcome of diffuse large B‐cell lymphoma in hepatitis C virus‐positive patients in LNH 93 and LNH 98 Groupe d'Etude des Lymphomes de l'Adulte programs. J Clin Oncol 200624953–960. [DOI] [PubMed] [Google Scholar]
- 29.Weng W ‐ K, Levy S. Hepatitis C virus (HCV) and lymphomagenesis. Leuk Lymphoma 2003441113–1120. [DOI] [PubMed] [Google Scholar]
- 30.Sansonno D, Tucci F A, Lauletta G.et al Hepatitis C virus productive infection in mononuclear cells from patients with cryoglobulinaemia. Clin Exp Immunol 2006147241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter R H, Fearon D T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992256105–107. [PubMed] [Google Scholar]
- 32.Petracca R, Falugi F, Galli G.et al Structure‐function analysis of hepatitis C virus envelope‐CD81 binding. J Virol 2000744824–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa D, Saletti G, De Gregorio E.et al Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus‐associated B lymphocyte disorders. Proc Natl Acad Sci USA 200510218544–18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanovski M, Silvestri F, Pozzato G.et al Somatic hypermutation, clonal diversity, and preferential expression of the VH 51 p1/VL kv325 immunoglobulin gene combination in hepatitis C virus‐associated immunocytomas. Blood 1998912433–2442. [PubMed] [Google Scholar]
- 35.De Re V, De Vita S, Marzotto A.et al Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus‐associated non‐Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor‐producing cells that occur mainly in type II cryoglobulinemia. Blood 2000963578–3584. [PubMed] [Google Scholar]
- 36.Machida K, Cheng K T ‐ N, Sung V M ‐ H.et al Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci USA 20041014262–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machida K, Cheng K T ‐ H, Pavio N.et al Hepatitis C virus E2‐CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol 2005798079–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofmann W P, Fernandez B, Herrmann E.et al Somatic hypermutation and mRNA expression levels of the BCL‐6 gene in patients with hepatitis C virus‐associated lymphoproliferative diseases. J Viral Hepat 200714484–491. [DOI] [PubMed] [Google Scholar]
- 39.Libra M, Capello D, Gloghini A.et al Analysis of aberrant somatic hypermutation (SHM) in non‐Hodgkin's lymphomas of patients with chronic HCV infection. J Pathol 200520687–91. [DOI] [PubMed] [Google Scholar]
- 40.Zignego A L, Ferri C, Giannelli F.et al Prevalence of bcl‐2 rearrangement in patients with hepatitis C virus‐related mixed cryoglobulinemia with or without B‐cell lymphomas. Ann Intern Med 2002137571–580. [DOI] [PubMed] [Google Scholar]
- 41.Kitay‐Cohen Y, Amiel A, Hilzenrat N.et al Bcl‐2 rearrangement in patients with chronic hepatitis C associated with essential mixed cryoglobulinemia type II. Blood 2000962910–2912. [PubMed] [Google Scholar]
- 42.Zuckerman E, Zuckerman T, Sahar D.et al bcl‐2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol 2001112364–369. [DOI] [PubMed] [Google Scholar]
- 43.Sasso E H, Martinez M, Yarfitz S L.et al Frequent joining of Bcl‐2 to a JH6 gene in hepatitis C virus‐associated t(14;18). J Immunol 20041733549–3556. [DOI] [PubMed] [Google Scholar]
- 44.Libra M, De Re V, De Vita S.et al Low frequency of bcl‐2 rearrangement in HCV‐associated non‐Hodgkin's lymphoma tissue. Leukemia 2003171433–1436. [DOI] [PubMed] [Google Scholar]
- 45.Libra M, De Re V, Gloghini A.et al Detection of bcl‐2 rearrangement in mucosa‐associated lymphoid tissue lymphomas from patients with hepatitis C virus infection. Haematologica 200489873–874. [PubMed] [Google Scholar]
- 46.Saadoun D, Suarez F, Lefrere F.et al Splenic lymphoma with villous lymphocytes, associated with type II cryoglobulinemia and HCV infection: a new entity? Blood 200510574–76. [DOI] [PubMed] [Google Scholar]
- 47.Hermine O, Lefrere F, Bronowicki J ‐ P.et al Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 200234789–94. [DOI] [PubMed] [Google Scholar]