Abstract
Although the morphology of the pathognomonic Reed–Sternberg cells of Hodgkin lymphoma (HL) was described over a century ago, it was not until recently that their origin from B lymphocytes was recognised. The demonstration that a proportion of cases of HL harbour the Epstein–Barr virus (EBV) and that its genome is monoclonal in these tumours suggests that the virus contributes to the development of HL in some cases. This review summarises current knowledge of the pathogenesis of HL with particular emphasis on the association with EBV.
Hodgkin lymphoma (HL) is characterised by the disruption of normal lymph node architecture and the presence of a minority of malignant Hodgkin/Reed–Sternberg (HRS) cells amid a background of non‐neoplastic cell populations comprising T‐ and B‐lymphocytes and other cell types.1 HRS cells and their reactive neighbouring cells cross‐talk via a complex of cytokine and cell contact dependent interactions; these probably include proliferative and anti‐apoptotic signals favouring tumour cell survival and expansion.2
The Revised European American Lymphoma (REAL)/World Health Organization (WHO) lymphoma classification3 divides HL into two major types: classical and nodular lymphocyte predominant HL (NLPHL) (table 1). Classical HL is further separated into four subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte depletion (LD) and a newly defined entity known as “lymphocyte rich classical” (LRC) HL. NLP and classical HL are separated on the basis of morphological, immunophenotypic and clinical differences.4 For example, HRS cells of NLPHL have different morphology, being referred to as lymphocytic and histiocytic (L&H) cells; they rarely express the classical HL markers CD15 or CD30, but regularly express B‐cell antigens such as CD20 and CD19, which are usually absent in HRS cells of classical HL.5
Table 1 Comparison of classical and nodular lymphocyte predominant Hodgkin lymphoma.
| REAL/WHO classification | Morphology/immunophenotype of HRS cells | EBV association | Ig status |
|---|---|---|---|
| Classical Hodgkin lymphoma | Typical HRS cells which are CD15+, CD20−, CD30+, CD45− | Positive or negative | Lack BCR expression |
| Nodular sclerosis | Destructive or non‐functional IGH rearrangements or loss of Ig‐specific transcription factors | ||
| Mixed cellularity | |||
| Lymphocyte depletion | |||
| Lymphocyte‐rich | |||
| Nodular lymphocyte‐predominant Hodgkin lymphoma | Atypical “popcorn” cells which are CD15−, CD20+, CD30−, CD45+, | Negative | Express BCR |
| Functional rearrangements of Ig genes | |||
| Evidence of intraclonal diversity indicating ongoing somatic hypermutation |
REAL/WHO, Revised European American Lymphoma (REAL)/World Health Organization; HRS, Hodgkin/Reed–Sternberg; EBV, Epstein–Barr virus; BCR, B‐cell receptor.
Origin of HRS cells
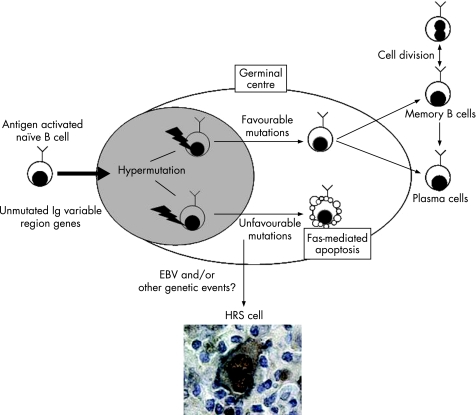
Early phenotypic studies suggested that HRS cells might be derived from macrophages, dendritic reticulum cells or granulocytes. However, the identification of clonally rearranged immunoglobulin (Ig) genes by PCR analysis of single HRS cells micromanipulated from HL tissues provided evidence not only of their malignant character but also of their origin from B cells.6 Tumour cells of both HL types carry somatic IGH mutations in the variable (V) region, indicating that they originate from germinal centre or post‐germinal centre B cells. However, only L&H cells of NLPHL demonstrate intraclonal V gene diversity due to ongoing mutation, suggesting that they originate from differentiating germinal centre B cells. In contrast, in 25% of classical HL, HRS cells carry non‐functional “crippling” mutations in the IGVH gene rearrangements, suggesting that HRS cells originate from pre‐apoptotic germinal centre B cells that were rescued from apoptosis by transforming events7 (fig 1).
Figure 1 Hodgkin/Reed–Sternberg (HRS) cells may originate from pre‐apoptotic germinal centre B cells. Naïve B cells are activated when they encounter cognate antigen. Activated B cells then migrate into B‐cell follicles, proliferate and differentiate into centroblasts, thus establishing germinal centres. Germinal centre (GC) B cells undergo somatic hypermutation of the V region genes; cells with unfavourable mutations are eliminated by Fas‐mediated apoptosis, whereas those carrying B‐cell receptor (BCR) with high affinity for antigen will survive and leave the germinal centre as memory B cells or plasma cells. GC B cells carrying non‐functional BCR should undergo apoptosis, but may be rescued by Epstein–Barr virus and/or still unknown genetic alterations. Such cells may be the progenitors of HRS cells.
The lack of expression of functional surface immunoglobulin (B‐cell receptor; BCR) is the hallmark of classical HL, and while in some cases this is certainly due to non‐functional or destructive rearrangements, other mechanisms can also account for the loss of functional BCR. For example, the loss of immunoglobulin‐specific transcription factors, BOB‐1, OCT2 and PU.1 in HRS cells has been reported.8,9,10,11 It has also been shown that, in a minority of cases, HRS cells carry mutations in the octamer region of the immunoglobulin gene promoter, which can prevent binding of Ig‐specific transcription factors.12,13 Epigenetic silencing can also suppress Ig transcription in HRS cells.14
Microarray profiling has revealed the down‐regulation in classical HL of B‐cell lineage gene expression, including many components of BCR signalling.15ID2, which causes a global down‐regulation of B‐cell genes by directly interacting and negatively regulating B‐cell specific transcription factors, such as E2A and PAX5, is strongly and uniformly expressed in HRS cells. Amplification or genomic gain of the ID2 locus has been reported in 50% of patients with HL and may account for its over‐expression in some cases.16,17 Activation of NOTCH1, which is expressed by HRS cells, can also suppress the B‐cell phenotype18 and could contribute to the characteristic loss of B‐cell identity. It has been suggested that the down‐regulation of B‐cell identity might allow HRS cells or their progenitors to escape the apoptosis that should occur in the absence of functional BCR, though there is no direct experimental evidence in support of this at present.19
Occasionally, HL tumours express T‐cell antigens, including granzyme B and T‐cell intracellular antigen (TIA)‐1. In some cases this has been shown to represent aberrant expression of T‐cell antigens by HRS cells that show evidence of IGH gene rearrangement and are thus assumed to be B cell in origin.20 However, in the same study a single HL case showed expression of T‐cell markers and also TCR gene rearrangements, indicating that at least a minority of HL tumours are genuinely of T‐cell origin.
EBV and Hodgkin lymphoma
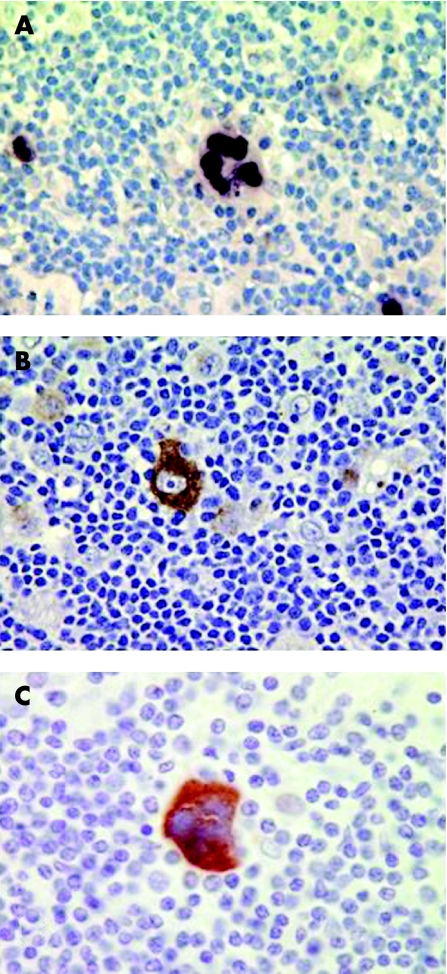
EBV is a ubiquitous human herpesvirus that infects over 90% of humans and persists for the lifetime of the person.21 First evidence that EBV might be involved in the pathogenesis of HL was provided by the detection of raised antibody titres to EBV antigens in HL patients when compared with other lymphoma patients,22 and furthermore, that these raised levels preceded the development of HL by several years.23 With the advent of cloned viral probes and Southern blot hybridisation methods, EBV DNA was initially detected in 20–25% of HL.24 In situ hybridisation provided the first demonstration of the existence of viral DNA in the HRS cells.25,26 Subsequently, the demonstration of the abundant EBV‐encoded RNAs (EBER1 and EBER2) in HRS cells provided a sensitive method for detecting latent infection in situ. This technique is now generally accepted as the “gold standard” for the detection of latent EBV infection in clinical samples27 (fig 2).
Figure 2 Gene expression in Epstein–Barr virus (EBV) associated Hodgkin lymphoma. (A) EBER expression (brown staining) in the nuclei of Hodgkin/Reed–Sternberg (HRS) cells. In situ hybridisation for EBER expression is the most reliable and sensitive method to detect the presence of latent EBV infection in clinical samples. (B) The latent membrane protein‐1 (LMP1) and (C) LMP2 are both highly expressed in EBV‐positive HRS cells. Not shown is the consistent expression of the EBV maintenance protein, EBNA1.
In EBV‐associated HL, viral genomes are found in monoclonal form, indicating that infection of the tumour cells occurred prior to their clonal expansion.25 In the majority of cases, EBV persists throughout the course of HL and is also found in multiple sites of HL.28
Although most EBV infections in children are asymptomatic or cause non‐specific symptoms, when primary infection is postponed until adolescence it can result in infectious mononucleosis (IM) in 50% of patients.21 The relative risk of developing HL in individuals with a history of IM, relative to those with no prior history, was shown to range between 2.0 and 5.0.29 It has recently been shown that the risk of EBV‐positive HL is increased four‐fold after IM, whereas the risk of EBV‐negative HL is not increased.30,31
The possibility that EBV may contribute to the pathogenesis of HL early in the transformation of the progenitor cells but is subsequently lost (“hit and run”), prompted the search for evidence of defective rearranged EBV DNA in tumours that by conventional testing (eg by detection of the EBERs) are virus negative. Such defective rearranged viral DNA has been previously detected in some cases of virus‐negative sporadic Burkitt lymphoma,32 and could result in partial elimination of EBV episomes from infected cells through the expression of BZLF1. In support of this possibility, Gan et al amplified sequences that span abnormally juxtaposed BamH1W and Z fragments (which characterise defective heterogeneous EBV DNA) from 2/24 EBER‐negative HL tumours in which the standard viral genome could not be detected.33 However, others using fluorescence in situ hybridisation (FISH) found no evidence of integrated EBV genomes in EBV‐negative HL.34 Furthermore, using quantitative PCR assays that spanned the whole genome, no evidence of deletion of EBV genomes in EBV‐positive HL, or retention of EBV genomes in EBV‐negative HL tissues, was found.35 Therefore, it seems unlikely that EBV contributes to the development of EBV‐negative HL.
The rate of detection of EBV in HL depends on factors such as country of residence, histological subtype, sex, ethnicity and age. EBV‐positive HL is less common in developed populations, with percentages of 20–50% for North American and European cases,27,36,37,38 57% for HL in China,39 but much higher rates in underdeveloped countries.40,41 The increased incidence of EBV‐positive HL in underdeveloped countries could be due to the existence of an underlying immunosuppression similar to that observed for African Burkitt lymphoma in a malaria‐infected population. This is supported by higher EBV‐positive rates in HL from HIV‐infected patients.42 Alternatively, the timing of EBV infection (which is likely to occur earlier in developing countries) might also be important.
EBV is more commonly associated with the MC subtype and less frequently with the other subtypes.43,44 Furthermore, it is now generally accepted that NLPHL is an EBV‐negative disease.45 HL in the older age groups and in children, especially boys under 10 years, has been shown to be more likely to be EBV‐associated than HL in young adults.46,47,48 This has led to the suggestion that HL consists of three disease entities: HL of childhood (EBV‐positive, MC type); HL of young adults (EBV‐negative, NS type); and HL of older adults (EBV‐positive, MC type).48 The higher proportion of EBV associated HL cases in older adults, is probably due to reduced immunosurveillance and increased viral reactivation occurring as a consequence of advancing years.49 The infrequent association of EBV with HL in young adulthood prompted the suggestion that a second virus might be involved, although there is little direct evidence to support this at present.50,51,52 EBV‐positive rates are generally higher in males than in females. EBV‐positive HL also affects more Asians and Hispanics than whites or blacks,46 and in the UK is more common in South Asian children compared with non‐South Asian children.53
Transforming events implicated in HRS pathogenesis
A number of cell signalling pathways are known to be aberrantly activated in HL. The PI3K/Akt pathway was recently shown to be constitutively activated in HL‐derived cell lines and in HRS cells from primary tumour tissues, where it was shown to contribute to the survival of these cells.54 The active phosphorylated form of ERK is also aberrantly expressed in cultured and primary HL cells; inhibition of the upstream MEK kinase inhibited the phosphorylation of ERK and the proliferation of HL cell lines.55 Kube et al have demonstrated the constitutive activation of STAT3 in HRS cells.56 STAT6 and STAT5a constitutive activation has also been reported.57,58 Amplification of the JAK2 locus is seen in some cases, providing a mechanistic explanation for the STAT activation.59 Furthermore, HRS cells also have constitutively activated AP‐1 with c‐Jun and JunB overexpression.60 AP‐1 activation was also observed in anaplastic large cell lymphoma (ALCL), but not in other lymphoma types. Constitutive activation of NF‐κB is a regular feature of HRS cells; inhibition of this pathway in HL cell lines leads to their increased sensitivity to apoptosis after growth factor withdrawal and impaired tumourigenicity in severe combined immunodeficiency (SCID) mice.61,62
Aberrant tyrosine kinase activity caused by various genetic alterations, such as activating point mutations, translocations or amplifications, frequently causes cellular transformation and has been observed in many different cancers. In mature B cells, several intracellular protein tyrosine kinases are essential for BCR signalling, whereas the expression of receptor tyrosine kinases (RTKs) is more limited; only expression of TRKA (tyrosine kinase receptor A) and MET have been described.63,64,65 In contrast, HL cells display aberrant activation of several RTKs, including PDGFRA, EPHB1, RON, TRKB, and TRKA, in the absence of activating mutations.66 Recently, it has been shown that aberrant tyrosine activity is more frequent in EBV‐negative HRS cells, suggesting that RTK signalling might partially replace the effects of EBV in HL pathogenesis.67
Under normal circumstances HRS cell progenitors lacking a functional BCR should be eliminated by Fas (CD95)‐mediated apoptosis in the germinal centre (GC). In vitro studies performed in our own laboratory and by others have demonstrated that HL‐derived cell lines are resistant to Fas‐mediated apoptosis.68,69,70,71 It is well established that HRS cells express Fas,70,72,73 and the rare occurrence of mutations in the FAS gene suggests that the general resistance of HRS cells to Fas‐induced death is due to defects further downstream in the death receptor pathway.74,75 c‐FLIP is a proximal negative regulator of CD95‐induced apoptosis that interferes with the formation of the death inducing signalling complex (DISC) required for death receptor induced death. A number of studies have shown that c‐FLIP is highly expressed in HL‐derived cell lines and primary HRS cells.68,69,71,75 We showed that c‐FLIP down‐regulation was accompanied by the spontaneous death of HL cells, even in the absence of challenge with Fas agonistic antibody, CH11.76 We also showed that HRS cells express Fas ligand (FasL); simultaneous knockdown of FasL and c‐FLIP expression did not cause HRS cell death, demonstrating that endogenous FasL could initiate death receptor signalling when c‐FLIP was silenced. Therefore, the down‐regulation of c‐FLIP unmasks a mechanism whereby HRS cells undergo self‐induced Fas‐mediated death. It should be noted that in a similar study, Mathas et al observed only a small increase in apoptosis following c‐FLIP knockdown alone, but much greater cell death after the addition of either CH11 or TRAIL following c‐FLIP knockdown.69 Taken together, these data suggest that abrogation of c‐FLIP expression might have therapeutic benefit in HL. HRS cells also have other defects in the apoptotic machinery. For example, they are defective in caspase 3‐mediated apoptosis because of their high level expression of XIAP, an inhibitor of apoptosis that binds and inhibits the proteolytic activity of caspases‐3, ‐7 and ‐9.77
Contribution of EBV genes to the pathogenesis of EBV‐positive Hodgkin lymphoma
EBV‐positive HRS cells exhibit a type II form of virus latency, virus gene expression being limited to the EBERs, Epstein–Barr nuclear antigen‐1 (EBNA1),78 latent membrane protein 1 (LMP1),43,44,79 LMP2,79,80 (fig 2), and the Bam H1A rightward transcripts (BARTs).79 EBV may contribute to the pathogenesis of HL before the HRS cell progenitor leaves the GC because it has been shown to rescue BCR‐negative human tonsillar GC cells from apoptosis, and because HRS cells with crippling IGH mutations are almost exclusively found in EBV‐positive patients.81,82,83,84,85
LMP1 induces many of the phenotypic changes observed in EBV‐infected B cells, including expression of the B‐cell activation markers, CD23 and CD40, interleukin (IL)‐10 production and up‐regulation of cell adhesion molecules such as ICAM1, LFA1 and LFA3. LMP1 also protects B cells from cell death by the up‐regulation of several anti‐apoptosis genes including BCL2, MCL1 and A20.86,87,88,89 LMP1 functions as a constitutively activated member of the tumour necrosis factor receptor (TNFR) superfamily activating cell signalling pathways in a ligand‐independent manner. LMP1 can engage the MAP kinase cascade resulting in the activation of ERK, JNK and p38 and can stimulate the JAK/STAT pathway.90,91,92,93,94 Two distinct functional domains referred to as C‐terminal activation regions 1 and 2 (CTAR1 and CTAR2) have been identified on the basis of their ability to activate these signalling pathways. CTAR1 activates NF‐κB mainly through the non‐canonical pathway (TRAF3/NIK/IKK‐alpha). In contrast, CTAR2 activates the canonical pathway by utilising TRAF6 and TAK1 to activate IKK‐beta.95 Although NF‐κB activation can be induced by LMP1 in EBV‐positive HRS cells, other routes to its activation must exist in EBV‐negative cases. IκBα mutations have been reported in HL and might be more frequent in EBV‐negative cases.96,97,98,99 Amplification of the NF‐κB/RelA locus at 2p13‐16 is also frequent in HL.100,101
LMP1 can induce expression of DNA methyltransferases and mediate hypermethylation of the E‐cadherin promoter in epithelial cells.102,103 We have recently shown that LMP1 can up‐regulate the Polycomb gene, BMI1, in HL cells.104 Bmi‐1 is a component of the polycomb repressive complex, PRC1; it is required for the initiation of gene silencing and induces lymphoid proliferation and the development of lymphomas in transgenic mice.105 We showed that BMI1 and LMP1 can regulate a shared subgroup of HL‐associated genes, including the ATM tumour suppressor.104 Therefore, it is likely that EBV mediates some its transforming effects through Bmi‐1.
The identification that a virus strain carrying a 30 bp deletion in the LMP1 gene was more tumourigenic than the prototype B95.8 LMP1106 led to numerous studies of the prevalence of this virus strain within EBV‐associated cancers, including HL. In general, virus strains carrying this 30 bp deletion occur with a similar frequency in virus‐positive tumour patients and in healthy donors from the same geographical region.107 The exception to this is HL,108 where some studies have shown an increased incidence of this deletion variant in HIV‐positive HL compared to HIV‐negative HL,109 and in paediatric HL compared to normal controls.110
LMP2A is also highly expressed in EBV‐positive HRS cells.79 LMP2A was shown to compete for the binding of the Src and Syk protein tyrosine kinases, thereby blocking BCR signalling and induction of lytic cycle in B cells.111 Paradoxically, expression of LMP2A in the B cells of transgenic mice abrogates normal B‐cell development, allowing immunoglobulin‐negative cells to colonise peripheral lymphoid organs,112 suggesting that LMP2A can provide a BCR‐like signal. LMP2A expression in transgenic mouse B cells down‐regulates expression of many of the B‐cell lineage genes that are absent or expressed at low levels in HRS cells (eg early B cell factor, PU.1, CD19, CD20).113 In addition, LMP2A expression induces the up‐regulation of genes involved in proliferation (eg MKI67, PCNA), protection from apoptosis (BCLXL, BIRC5 (survivin)) and suppression of cell‐mediated immunity (eg IL13R, EBI3).113
We recently showed that EBV infection of HL cells up‐regulates autotaxin, a secreted tumour‐associated factor with lysophospholipase‐D activity. Up‐regulation of autotaxin increased the generation of lysophosphatidic acid (LPA) and led to the enhanced growth and survival of Hodgkin lymphoma cells.114 This is the first demonstration that virus infection leads directly to the synthesis of the growth‐promoting lipid LPA and could represent a more general pathway utilised by herpesviruses during their normal life cycle or during the initiation and maintenance of virus‐associated tumours. At this time it is unknown which EBV gene is responsible for this effect.
Studies of B cell subsets from healthy carriers have revealed that EBV persists in the peripheral blood in IgD‐negative memory B cells (CD19+, CD23−, CD80/B7−) where viral protein expression may be restricted to LMP2A.115 Following primary infection of naïve or memory B cells, the virus expresses the EBNA2‐dependent lymphoblastoid growth programme. The detection of both LMP1 and LMP2 in purified tonsillar memory B cells and germinal centre B cells116 suggests that these viral proteins, through surrogate T‐cell help (LMP1) and BCR engagement (LMP2A), provide the necessary signals for EBV‐infected B cells to undergo antigen‐independent proliferation in the germinal centre, in turn leading to replenishment of the pool of EBV‐positive memory B cells. However, the recent demonstration that EBV‐positive memory B cells are antigen selected suggests that these cells do not arise from a B‐cell differentiation programme driven only by the virus. In this context LMP1 and LMP2 expression might favour the selection of EBV‐infected B cells in the highly competitive antigen‐dependent environment of the GC.117 Together with as yet undefined cellular alterations, LMP1 and LMP2 could also favour the neoplastic transformation of GC B cells, leading to the development of EBV‐positive HL.
Although this model is appealing, other studies suggest that the influence of LMP1 during B‐cell differentiation is more complicated. LMP1 expression cannot be detected within GC of lymphoid tissues by immunohistochemistry118 and has been shown to induce extra‐follicular B‐cell differentiation but not GC formation.119 Therefore, it is possible that EBV accesses the memory‐B‐cell pool following an extra‐follicular B‐cell maturation programme which is driven by LMP1, and not through the conventional GC stage. CD40 can control the exit of B cells from the GC, and inhibit their further differentiation into plasma cells.120 If LMP1 has similar effects on GC B‐cell differentiation, an LMP1‐expressing post‐GC/pre‐plasma cell may emerge from which the HRS cell develops. This would be consistent with the finding that HRS cells often express some of the markers of plasma cell differentiation; and also with the observation that the completion of plasma cell differentiation may be prejudicial to the maintenance of viral latency because it can result in the reactivation of viral replication.121
Failure of the immune response to eliminate EBV‐infected HRS cells and attempts to develop immunotherapeutic strategies
LMP2A and LMP1 are targets for cytotoxic T lymphocytes (CTLs) in association with different MHC class I restriction elements in vitro.122,123 In vitro HL cells can process and present epitopes from LMP1 and LMP2A in the context of multiple class I alleles and are sensitive to lysis by EBV‐specific CTLs.131,132 However, EBV‐infected HRS cells survive in vivo. HRS cells express the thymus and activated regulated chemokine (TARC), IL‐10, IL‐13 and TGFβ which could counteract EBV‐specific CTL responses.124,125,126,127 Surprisingly, EBV‐positive cases of HL have been shown to contain more activated CTLs and express higher levels of MHC class I than EBV‐negative cases.128,129,130,131
EBV‐specific CTLs can be generated from patients with advanced HL, albeit at lower frequency than normal controls.133 These EBV‐specific CTLs survived and had antiviral activity in vivo; however, despite the resolution of some symptoms and the stabilisation of disease, all HL patients failed to recover from their disease. Successful CTL therapy for EBV‐positive HL will require not only EBV‐specific CTL with improved cytotoxicity, but probably also modification of the tumour microenvironment to remove the inhibitory barriers which inhibit CTL function. Antigen presentation systems may improve the cytolytic activity of CTLs. For example, exposure to AdE1‐LMPpoly (a replication‐deficient adenoviral system which encodes a glycine alanine repeat‐deleted EBNA1 covalently linked to multiple CD8+ T cell epitopes from LMP1 and LMP2) was effective in expanding T cells specific for LMP1, LMP2A, and EBNA1, across a range of HLA types.134 A recombinant poxvirus vaccine encoding a polypeptide protein comprising six HLA A2 restricted epitopes derived from LMP1 (“LMP1 polyepitope vaccine”), can generate stronger anti‐LMP1 responses.136 CTLs generated by exposure to LMP2A‐expressing dendritic cells demonstrate increased cytotoxicity.135
There are a number of possibilities to alleviate the CTL inhibitory effect of the microenvironment,137,138 but the depletion of regulatory T cells (Tregs) is likely to be of prime importance. Recent studies show that both tumour infiltrating lymphocytes and peripheral blood mononuclear cells from HL patients contain many Tregs.139 A recent study showed that Tregs which express LAG‐3 are more frequent in EBV‐positive compared with EBV‐negative HL.139 Furthermore, the level of LAG‐3 expression on the tumour‐infiltrating lymphocytes was coincident with impairment of T‐cell activity against LMP1 and LMP2, whereas deletion of CD4+ LAG‐3+ T‐cells enhanced LMP‐specific immunity.
Take‐home messages
Epstein‐Barr virus (EBV) is associated with only a proportion of cases of Hodgkin lymphoma (HL), where the virus is present in the tumour cell populations. The virus‐negative and virus‐positive forms of the disease are morphologically similar but appear to differ in terms of their precise underlying molecular changes.
EBV contributes to the aberrant activation of key signalling pathways in HL; understanding how EBV subverts cellular physiology can also inform our understanding of EBV‐negative HL.
The development of EBV‐associated HL may be regarded as an unintentional consequence of the normal viral life cycle which is designed to enable EBV to persist in the B cell pool of normal carriers.
Understanding the mechanisms by which EBV transforms cells and escapes the immune system will be important for the development of strategies to treat patients with EBV‐associated malignancies.
Summary
A proportion of HL tissues harbour EBV within tumour cells. Superficially at least, there are surprisingly few differences between EBV‐positive and EBV‐negative HRS cells. Emerging evidence suggests that while EBV is able to subvert cellular processes in favour of growth and survival, cellular genetic events are required to do this when EBV is absent. The challenge is to unravel this complexity by detailed consideration of the function of latent EBV genes in the appropriate cellular context. It is hoped that this approach will reveal more fundamental aspects of HL pathogenesis and pave the way for more targeted therapies for HL patients.
Footnotes
Funding: The Leukaemia Research Fund, Cancer Research UK and the Association for International Cancer Research provided support for this study.
Competing interests: None declared.
References
- 1.Harris N L, Jaffe E S, Stein H.et al A Revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994841361–1392. [PubMed] [Google Scholar]
- 2.Pinto A, Gattei V, Zagonel V.et al Hodgkin's disease: a disorder of dysregulated cellular cross‐talk. Biotherapy 199810309–320. [DOI] [PubMed] [Google Scholar]
- 3.Harris N L, Jaffe E S, Diebold J.et al The World Health Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues. Ann Oncol 1999101419–1432. [DOI] [PubMed] [Google Scholar]
- 4.Küppers R, Yahalom J, Josting A. Advances in biology, diagnostics, and treatment of Hodgkin's disease. Biol Blood Marrow Transplant 20061266–76. [DOI] [PubMed] [Google Scholar]
- 5.Buettner M, Greiner A, Avramidou A.et al Evidence of abortive plasma cell differentiation in Hodgkin and Reed‐Sternberg cells of classical Hodgkin lymphoma. Hematol Oncol 200523127–132. [DOI] [PubMed] [Google Scholar]
- 6.Küppers R, Rajewsky K, Zhao M.et al Hodgkin disease: Hodgkin and Reed‐Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci USA 19949110962–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanzler H, Kuppers R, Hansmann M L.et al Hodgkin and Reed‐Sternberg cells in Hodgkin's disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med 19961841495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Re D, Muschen M, Ahmadi T.et al Oct‐2 and Bob‐1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res 2001612080–2084. [PubMed] [Google Scholar]
- 9.Jundt F, Kley K, Anagnostopoulos I.et al Loss of PU.1 expression is associated with defective immunoglobulin transcription in Hodgkin and Reed‐Sternberg cells of classical Hodgkin disease. Blood 2002993060–3062. [DOI] [PubMed] [Google Scholar]
- 10.Torlakovic E, Tierens A, Dang H D.et al The transcription factor PU.1, necessary for B‐cell development is expressed in lymphocyte predominance, but not classical Hodgkin's disease. Am J Pathol 20011591807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marafioti T, Hummel M, Foss H ‐ D.et al Hodgkin and Reed‐Sternberg cells represent an expansion of a single clone originating from a germinal centre B‐cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000951443–1450. [PubMed] [Google Scholar]
- 12.Jox A, Zander T, Kuppers R.et al Somatic mutations within the untranslated regions of rearranged Ig genes in a case of classical Hodgkin's disease as a potential cause for the absence of Ig in the lymphoma cells. Blood 1990933964–3972. [PubMed] [Google Scholar]
- 13.Theil J, Laumen H, Marafioti T.et al Defective octamer‐dependent transcription is responsible for silenced immunoglobulin transcription in Reed‐Sternberg cells. Blood 2001973191–3196. [DOI] [PubMed] [Google Scholar]
- 14.Ushmorov A, Ritz O, Hummel M.et al Epigenetic silencing of the immunoglobulin heavy‐chain gene in classical Hodgkin lymphoma‐derived cell lines contributes to the loss of immunoglobulin expression. Blood 20041043326–3334. [DOI] [PubMed] [Google Scholar]
- 15.Schwering I, Brauninger A, Klein U.et al Loss of the B‐lineage‐specific gene expression program in Hodgkin and Reed‐Sternberg cells of Hodgkin lymphoma. Blood 20031011505–1512. [DOI] [PubMed] [Google Scholar]
- 16.Renne C, Martin‐Subero J I, Eickernjager M.et al Aberrant expression of ID2, a suppressor of B‐cell specific gene expression in Hodgkin's lymphoma. Am J Pathol 2006169655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathas S, Janz M, Hummel F.et al Intrinsic inhibition of transcription factor E2A by HLH proteins ABF‐1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol 20067207–215. [DOI] [PubMed] [Google Scholar]
- 18.Radtke F, Wilson A, Mancini S J.et al Notch regulation of lymphocyte development and function. Nat Immunol 20045247–253. [DOI] [PubMed] [Google Scholar]
- 19.Kuppers R, Hansmann M. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol 200537511–517. [DOI] [PubMed] [Google Scholar]
- 20.Müschen M, Rajewsky K, Bräuninger A.et al Rare occurrence of classical Hodgkin's disease as a T cell lymphoma. J Exp Med 2000191387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J I. Epstein‐Barr virus infection. N Engl J Med 2000343481–492. [DOI] [PubMed] [Google Scholar]
- 22.Levine P H, Ablashi D V, Berard C W.et al Elevated antibody titers to Epstein‐Barr virus in Hodgkin's disease. Cancer 197127416–421. [DOI] [PubMed] [Google Scholar]
- 23.Mueller N, Evans A, Harris N L.et al Hodgkin's disease and Epstein‐Barr virus: altered antibody pattern before diagnosis. N Engl J Med 1989320689–695. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L M, Strickler J G, Warnke R A.et al Epstein‐Barr viral DNA in tissues of Hodgkin's disease. Am J Pathol 198712986–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Anagnostopoulos I, Herbst H, Niedobitek G.et al Demonstration of monoclonal EBV genomes in Hodgkin's disease and Ki‐1positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood 198974810–816. [PubMed] [Google Scholar]
- 26.Weiss L M, Movahed L A, Warnke R A.et al Detection of Epstein‐Barr viral genomes in Reed‐Sternberg cells of Hodgkin's disease. N Engl J Med 1989320502–506. [DOI] [PubMed] [Google Scholar]
- 27.Wu T C, Mann R B, Charache P.et al Detection of EBV gene expression in Reed‐Sternberg cells of Hodgkin's disease. Int J Cancer 199046801–804. [DOI] [PubMed] [Google Scholar]
- 28.Coates P J, Slavin G, D'Ardenne A J. Persistence of Epstein‐Barr virus in Reed‐Sternberg cells throughout the course of Hodgkin's disease. J Pathol 1991164291–297. [DOI] [PubMed] [Google Scholar]
- 29.Gutensohn N, Cole P. Epidemiology of Hodgkin's disease. Semin Oncol 1980792–102. [PubMed] [Google Scholar]
- 30.Hjalgrim H, Askling J, Rostgaard K.et al Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med 20033491324–1332. [DOI] [PubMed] [Google Scholar]
- 31.Hjalgrim H, Ekstrom Smedby K, Rostgaard K.et al Infectious mononucleosis, childhood social environment and risk of Hodgkin lymphoma. Cancer Res 2007672382–2388. [DOI] [PubMed] [Google Scholar]
- 32.Razzouk B I, Srinivas S, Sample C E.et al Epstein‐Barr virus DNA recombination and loss in sporadic Burkitt's lymphoma. J Infect Dis 1996173529–535. [DOI] [PubMed] [Google Scholar]
- 33.Gan Y J, Razzouk B I, Su T.et al A defective, rearranged Epstein‐Barr virus genome in EBER‐negative and EBER‐positive Hodgkin's disease. Am J Pathol 2002160781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staratschek‐Jox A, Kotkowski S, Belge G.et al Detection of Epstein‐Barr virus in Hodgkin‐Reed‐Sternberg cells: no evidence for the persistence of integrated viral fragments in latent membrane protein‐1 (LMP‐1)‐negative classical Hodgkin's disease. Am J Pathol 2000156209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallagher A, Perry J, Freeland J.et al Hodgkin lymphoma and Epstein‐Barr virus (EBV): no evidence to support hit‐and‐run mechanism in cases classified as non‐EBV‐associated. Int J Cancer 2003104624–630. [DOI] [PubMed] [Google Scholar]
- 36.Weiss L M, Chen Y ‐ Y, Liu X ‐ F.et al Epstein‐Barr virus and Hodgkin's disease: a correlative in situ hybridization and polymerase chain reaction study. Am J Pathol 19911391259–1265. [PMC free article] [PubMed] [Google Scholar]
- 37.Herbst H, Steinbrecher E, Niedobitek G.et al Distribution and phenotype of Epstein‐Barr virus‐harboring cells in Hodgkin's disease. Blood 199280484–491. [PubMed] [Google Scholar]
- 38.Hummel M, Anagnostopoulos I, Dallenbach F.et al EBV infection patterns in Hodgkin's disease and normal lymphoid tissue: expression and cellular localization of gene products. Brit J Haematol 199282689–694. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X ‐ G, Hamilton‐Dutoit S J, Yan Q ‐ H.et al The association between Epstein‐Barr virus and Chinese Hodgkin's disease. Int J Cancer 199355359–363. [DOI] [PubMed] [Google Scholar]
- 40.Chang K L, Albujar P F, Chen Y ‐ Y.et al High prevalence of Epstein‐Barr virus in the Reed‐Sternberg cells of Hodgkin's disease occurring in Peru. Blood 199381496–501. [PubMed] [Google Scholar]
- 41.Weinreb M, Day P J R, Niggli F.et al The consistent association between Epstein‐Barr virus and Hodgkin's disease in children in Kenya. Blood 1996873828–3836. [PubMed] [Google Scholar]
- 42.Uccini S, Monardo F, Stoppacciaro A.et al High frequency of Epstein‐Barr virus‐genome detection in Hodgkin's disease of HIV‐positive patients. Int J Cancer 199046581–585. [DOI] [PubMed] [Google Scholar]
- 43.Pallesen G, Hamilton‐Dutoit S J, Rowe M.et al Expression of Epstein‐Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet 1991337320–322. [DOI] [PubMed] [Google Scholar]
- 44.Murray P G, Young L S, Rowe M.et al Immunohistochemical demonstration of the Epstein‐Barr virus‐encoded latent membrane protein in paraffin sections of Hodgkin's disease. J Pathol 19921661–5. [DOI] [PubMed] [Google Scholar]
- 45.Chan W C. Cellular origin of nodular lymphocyte‐predominant Hodgkin's lymphoma: immunophenotypic and molecular studies. Semin Hematol 199936242–252. [PubMed] [Google Scholar]
- 46.Glaser S L, Lin R J, Stewart S L.et al Epstein‐Barr virus‐associated Hodgkin's disease: epidemiologic characteristics in international data. Int J Cancer 199770375–382. [DOI] [PubMed] [Google Scholar]
- 47.Jarrett R F, Gallagher A, Jones D B.et al Detection of Epstein‐Barr virus genomes in Hodgkin's disease: relation to age. J Clin Pathol 199144844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong A A, Alexander F E, Cartwright R.et al Epstein‐Barr virus and Hodgkin's disease: further evidence for the three disease hypothesis. Leukemia 1998121272–1276. [DOI] [PubMed] [Google Scholar]
- 49.Khan G, Lake A, Shield L.et al Phenotype and frequency of Epstein‐Barr virus‐infected cells in pre‐treatment blood samples from patients with Hodgkin lymphoma. Br J Haematol 2005129511–519. [DOI] [PubMed] [Google Scholar]
- 50.Wilson K S, Gallagher A, Freeland J M.et al Viruses and Hodgkin lymphoma: no evidence of polyomavirus genomes in tumor biopsies. Leuk Lymphoma 2006471315–1321. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher A, Perry J, Shield L.et al Virus and Hodgkin disease: no evidence of novel herpesviruses in non‐EBV‐associated lesions. Int J Cancer 2002101259–264. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong A A, Shield L, Gallagher A.et al Lack of involvement of known oncogenic DNA viruses in Epstein‐Barr virus‐negative Hodgkin's disease. Br J Cancer 1998771045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flavell K J, Biddulph J P, Powell J E.et al South Asian ethnicity and material deprivation increase the risk of Epstein‐Barr virus infection in childhood Hodgkin's disease. Br J Cancer 200185350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutton A, Reynolds G M, Dawson C W.et al Constitutive activation of phosphatidyl‐inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase amd mTOR. J Pathol 2005205498–506. [DOI] [PubMed] [Google Scholar]
- 55.Zheng B, Fiumara P, Li Y V.et al MEK/ERK pathway is aberrantly active in Hodgkin's disease: a signalling pathway shared by CD30, CD40 and RANK that regulates cell proliferation and survival. Blood 20031021019–1027. [DOI] [PubMed] [Google Scholar]
- 56.Kube D, Holtick U, Vockerodt M.et al STAT3 is constitutively activated in Hodgkin cell lines. Blood 200198762–770. [DOI] [PubMed] [Google Scholar]
- 57.Skinnider B F, Elia A J, Gascoyne R D.et al Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed‐Sternberg cells of Hodgkin lymphoma. Blood 200299618–626. [DOI] [PubMed] [Google Scholar]
- 58.Hinz M, Lemke P, Anagnostopoulos I.et al Nuclear factor kappaB‐dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med 2002196605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joos S, Granzow M, Holtgreve‐Grez H.et al Hodgkin's lymphoma cell lines are characterized by frequent aberrations on chromosomes 2p and 9p including REL and JAK2. Int J Cancer 2003103489–495. [DOI] [PubMed] [Google Scholar]
- 60.Mathas S, Hinz M, Anagnostopoulos I.et al Aberrantly expressed c‐Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF‐kappa B. EMBO J 2001214104–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bargou R C, Leng C, Krappmann D.et al High‐level nuclear NF‐kappa B and Oct‐2 is a common feature of cultured Hodgkin/Reed‐Sternberg cells. Blood 1996874340–4347. [PubMed] [Google Scholar]
- 62.Bargou R C, Emmerich F, Krappmann D.et al Constitutive nuclear factor‐kappaB‐RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest 19971002961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kronfeld I, Kazimirsky G, Gelfand E W.et al NGF rescues human B lymphocytes from anti‐IgM induced apoptosis by activation of PKCζ. Eur J Immunol 200232136–143. [DOI] [PubMed] [Google Scholar]
- 64.Skibinski G, Skibinska A, James K. The role of hepatocyte growth factor and its receptor c‐met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 2001102506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torcia M, Bracci‐Laudiero L, Lucibello M.et al Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell 199685345–356. [DOI] [PubMed] [Google Scholar]
- 66.Renne C, Willenbrock K, Kuppers R.et al Autocrine‐ and paracrine‐activated receptor tyrosine kinases in classic Hodgkin's lymphoma. Blood 20051054051–4059. [DOI] [PubMed] [Google Scholar]
- 67.Renné C, Hinsch N, Willenbrock K.et al The aberrant coexpression of several receptor tyrosine kinases is largely restricted to EBV‐negative cases of classical Hodgkin's lymphoma. Int J Cancer 20071202504–2509. [DOI] [PubMed] [Google Scholar]
- 68.Dutton A, Burns A T, Young L S.et al Targeting cellular FLICE‐like inhibitory protein as a novel approach to the treatment of Hodgkin's lymphoma. Expert Rev Anticancer Ther 20066911–919. [DOI] [PubMed] [Google Scholar]
- 69.Mathas S, Lietz A, Anagnostopoulos I.et al C‐Flip mediates resistance of Hodgkin/Reed‐Sternberg cells to death receptor‐induced apoptosis. J Exp Med 20041991041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Re D, Hofmann A, Wolf J.et al Cultivated H‐RS cells are resistant to CD95L‐mediated apoptosis despite expression of wild‐type CD95. Exp Hematol 20002831–35. [DOI] [PubMed] [Google Scholar]
- 71.Thomas R K, Kallenborn A, Wickenhauser C. Constitutive expression of c‐FLIP in Hodgkin and Reed‐Sternberg cells. Am J Pathol 20021601521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xerri L, Carbuccia N, Parc P.et al Frequent expression of Fas/APO‐1 in Hodgkin's disease and anaplastic large cell lymphomas. Histopathology 199527235–241. [DOI] [PubMed] [Google Scholar]
- 73.Metkar S S, Naresh K N, Redkar A A.et al Expression of Fas and Fas ligand in Hodgkin's disease. Leuk Lymphoma 199933521–530. [DOI] [PubMed] [Google Scholar]
- 74.Muschen M, Re D, Brauninger A.et al Somatic mutations of the CD95 gene in Hodgkin and Reed‐Sternberg cells. Cancer Res 2000605640–5643. [PubMed] [Google Scholar]
- 75.Maggio E M, Van Der Berg A, De Jong D.et al Low frequency of Fas mutations I Reed‐Sternberg cells of Hodgkin's lymphoma. Am J Pathol 200316229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dutton A, O'Neil J D, Milner A E.et al Expression of the cellular FLICE‐inhibitory protein (c‐FLIP) protects Hodgkin's lymphoma cells from autonomous Fas‐mediated death. Proc Natl Acad Sci USA 20041016611–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kashkar H, Haefs C, Shin H.et al XIAP‐mediated caspase inhibition in Hodgkin's lymphoma‐derived B cells.J Exp Med 2003198341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grässer F A, Murray P G, Kremmer E.et al Monoclonal antibodies directed against the Epstein‐Barr virus‐encoded nuclear antigen 1 (EBNA 1): immunohistologic detection of EBNA 1 in the malignant cells of Hodgkin's disease. Blood 1994843792–3798. [PubMed] [Google Scholar]
- 79.Deacon E M, Pallesen G, Niedobitek G.et al Epstein‐Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med 1993177339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niedobitek G, Kremmer E, Herbst H.et al Immunohistochemical detection of the Epstein‐Barr virus‐encoded latent membrane protein 2A in Hodgkin's disease and infectious mononucleosis. Blood 1997901664–1672. [PubMed] [Google Scholar]
- 81.Vockerodt M, Belge G, Kube D.et al An unbalanced translocation involving chromosome 14 is the probable cause for loss of potentially functional rearranged immunoglobulin heavy chain genes in the Epstein‐Barr virus‐positive Hodgkin's lymphoma‐derived cell line L591. Br J Haematol 2002119640–646. [DOI] [PubMed] [Google Scholar]
- 82.Brauninger A, Schmitz R, Bechtel D.et al Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer 20061181853–1861. [DOI] [PubMed] [Google Scholar]
- 83.Bechtel D, Kurth J, Unkel C.et al Transformation of BCR‐deficient germinal centre B cells by EBV supports a major role of the virus in the pathogenesis of Hodgkin's and posttransplant lymphoma. Blood 20051064345–4350. [DOI] [PubMed] [Google Scholar]
- 84.Chaganti S, Bell A I, Beque‐Pastor N.et al Epstein‐Barr virus infection in vitro can rescue germinal centre B cells with inactivated immunoglobulin genes. Blood 20051064249–4252. [DOI] [PubMed] [Google Scholar]
- 85.Mancao C, Altmann M, Jungnickel B.et al Rescue of ‘crippled' germinal centre B cells from apoptosis by Epstein‐Barr virus. Blood 20051064339–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henderson S, Rowe M, Gregory C.et al Induction of bcl‐2 expression by Epstein‐Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 1991651107–1115. [DOI] [PubMed] [Google Scholar]
- 87.Laherty C D, Hu H M, Opipari A W.et al The Epstein‐Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem 199226724157–24160. [PubMed] [Google Scholar]
- 88.Wang S, Rowe M, Lundgren E. Expression of the Epstein Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl‐2 homologue Mcl‐1 levels in B‐cell lines. Cancer Res 1996564610–4613. [PubMed] [Google Scholar]
- 89.Rowe M, Peng‐Pilon M, Huen D S.et al Upregulation of bcl‐2 by the Epstein‐Barr virus latent membrane protein LMP1: a B‐cell‐specific response that is delayed relative to NF‐kappa B activation and to induction of cell surface markers. J Virol 1994685602–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eliopoulos A G, Gallagher N J, Blake S M.et al Activation of the p38 mitogen‐activated protein kinase pathway by Epstein‐Barr virus‐encoded latent membrane protein 1 coregulates interleukin‐6 and interleukin‐8 production. J Biol Chem 199927416085–16096. [DOI] [PubMed] [Google Scholar]
- 91.Eliopoulos A G, Young L S. Activation of the cJun N‐terminal kinase (JNK) pathway by the Epstein‐Barr virus‐encoded latent membrane protein 1 (LMP1). Oncogene 1998161731–1742. [DOI] [PubMed] [Google Scholar]
- 92.Gires O, Kohlhuber F, Kilger E.et al Latent membrane protein 1 of Epstein‐Barr virus interacts with JAK3 and activates STAT proteins. EMBO J 1999183064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts M L, Cooper N R. Activation of a ras‐MAPK‐dependent pathway by Epstein‐Barr virus latent membrane protein 1 is essential for cellular transformation. Virology 199824093–99. [DOI] [PubMed] [Google Scholar]
- 94.Kieser A, Kilger E, Gires O.et al Epstein‐Barr virus latent membrane protein‐1 triggers AP‐1 activity via the c‐Jun N‐terminal kinase cascade. EMBO J 1997166478–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu L, Nakano H, Wu Z. The CTAR2 domain of the Epstein‐Barr virus‐encoded latent membrane protein 1 activated NF‐kappa B through TRAF6 and TAK1. J Biol Chem 20052812162–2169. [DOI] [PubMed] [Google Scholar]
- 96.Emmerich F, Meiser M, Hummel M.et al Overexpression of I kappa B alpha without inhibition of NF‐kappaB activity and mutations in the I kappa B alpha gene in Reed‐Sternberg cells. Blood 1999943129–3134. [PubMed] [Google Scholar]
- 97.Cabannes E, Khan G, Aillet F.et al Mutations in the IkBa gene in Hodgkin's disease suggest a tumour suppressor role for IkappaBalpha. Oncogene 1999183063–3070. [DOI] [PubMed] [Google Scholar]
- 98.Jungnickel B, Staratschek‐Jox A, Bräuninger A.et al Clonal deleterious mutations in the IκBα gene in the malignant cells in Hodgkin's lymphoma. J Exp Med 2000191395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jarrett R F, Lake A, Andrew L.et al Somatic IkBa mutations are a frequent occurrence in Hodgkin's lymphoma [abstract]. Blood 20021004333 [Google Scholar]
- 100.Martin‐Subero J I, Gesk S, Harder L.et al Recurrent involvement of the REL and BCL11A loci in classical Hodgkin lymphoma. Blood 2002991474–1477. [DOI] [PubMed] [Google Scholar]
- 101.Barth T F, Martin‐Subero J I, Joos S.et al Gains of 2p involving the REL locus correlate with nuclear c‐Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 20031013681–3686. [DOI] [PubMed] [Google Scholar]
- 102.Tsai C N, Tsai C L, Tse K P.et al The Epstein‐Barr virus oncogene product, latent membrane protein1, induces the down‐regulation of E‐cadherin gene expression via activation of DNA methyltransferases. PNAS 20029910084–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 20066846–856. [DOI] [PubMed] [Google Scholar]
- 104.Dutton A, Woodman C B, Chukwuma M B.et al Bmi‐1 is induced by the Epstein‐Barr oncogene LMP1 and regulates the expression of viral target genes in Hodgkin lymphoma cells. Blood 20061092597–2603. [DOI] [PubMed] [Google Scholar]
- 105.Tsai C L, Li H P, Lu Y J.et al Activation of DNA methyltransferase 1 by EBV LMP1 involves c‐Jun NH2‐terminal kinase signalling. Cancer Res 20066611668–11676. [DOI] [PubMed] [Google Scholar]
- 106.Hu L F, Chen F, Zheng X.et al Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene 199381575–1583. [PubMed] [Google Scholar]
- 107.Khanim F, Yao Q ‐ Y, Niedobitek G.et al Analysis of Epstein‐Barr virus gene polymorphisms in normal donors and in virus‐associated tumors from different geographic locations. Blood 1996883491–3501. [PubMed] [Google Scholar]
- 108.Knecht H, Bachmann E, Brousset P.et al Deletions within the LMP1 oncogene of Epstein‐Barr virus are clustered in Hodgkin's disease and identical to those observed in nasopharyngeal carcinoma. Blood 1993822937–2942. [PubMed] [Google Scholar]
- 109.Bellas C, Santon A, Manzanal A.et al Pathological, immunological, and molecular features of Hodgkin's disease associated with HIV infection comparison with ordinary Hodgkin's disease. Am J Surg Pathol 1996201520–1524. [DOI] [PubMed] [Google Scholar]
- 110.Santon A, Martin C, Manzanal A I.et al Paediatric Hodgkin's disease in Spain: association with Epstein‐Barr virus strains carrying latent membrane protein‐1 oncogene deletions and high frequency of dual infections. Br J Haematol 1998103129–136. [DOI] [PubMed] [Google Scholar]
- 111.Miller C L, Burkhardt A L, Lee J H.et al Integral membrane protein 2 of Epstein‐Barr virus regulates reactivation from latency through dominant negative effects on protein‐tyrosine kinases. Immunity 19952155–166. [DOI] [PubMed] [Google Scholar]
- 112.Caldwell R G, Wilson J B, Anderson S J.et al Epstein‐Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 19989405–411. [DOI] [PubMed] [Google Scholar]
- 113.Portis T, Dyck P, Longnecker R. Epstein‐Barr Virus (EBV) LMP2A induces alterations in gene transcription similar to those observed in Reed‐Sternberg cells of Hodgkin lymphoma. Blood 20031024166–4178. [DOI] [PubMed] [Google Scholar]
- 114.Baumforth K R N, Flavell J R, Reynolds G M.et al Induction of autotoxin by the Esptein‐Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood 20051062138–2146. [DOI] [PubMed] [Google Scholar]
- 115.Babcock G J, Decker L L, Volk M.et al EBV persistence in memory B cells in vivo. Immunity 19989395–404. [DOI] [PubMed] [Google Scholar]
- 116.Babcock G J, Thorley‐Lawson D A. Tonsillar memory B cells, latently infected with Epstein‐Barr virus, express the restricted pattern of latent genes previously found only in Epstein‐Barr virus‐associated tumours. Proc Natl Acad Sci USA 20009712250–12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thorley‐Lawson D A. EBV the prototypical human tumor virus‐just how bad is it? J Allergy Clin Immunol 2005116251–256. [DOI] [PubMed] [Google Scholar]
- 118.Niedobitek G, Agathanggelou A, Herbst H.et al Epstein‐Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV‐infected cells. J Pathol 1997182151–159. [DOI] [PubMed] [Google Scholar]
- 119.Uchida J, Yasui T, Takaoka‐Scichijo Y.et al Mimicry of CD40 signals by Epstein‐Barr virus LMP1 in B lymphocyte responses. Science 1999286300–303. [DOI] [PubMed] [Google Scholar]
- 120.Basso K, Klein U, Niu H.et al Tracking CD40 signalling during germinal centre development. Blood 20041044088–4096. [DOI] [PubMed] [Google Scholar]
- 121.Laichalk L L, Thorley‐Lawson D A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein‐Barr virus in vivo. J Virol 2005791296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khanna R, Burrows S R, Nicholls J.et al Identification of cytotoxic T cell epitopes within Epstein‐Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype‐restricted immune recognition of EBV‐infected cells by LMP1‐specific cytotoxic T lymphocytes. Eur J Immunol 199828451–458. [DOI] [PubMed] [Google Scholar]
- 123.Lee S P, Tierney R J, Thomas W A.et al Conserved CTL epitopes within EBV latent membrane protein 2: a potential target for CTL‐based tumor therapy J Immunol 19971583325–3334. [PubMed] [Google Scholar]
- 124.Herbst H, Foss H D, Samol J.et al Frequent expression of interleukin‐10 by Epstein‐Barr virus‐harboring tumor cells of Hodgkin's disease. Blood 1996872918–2929. [PubMed] [Google Scholar]
- 125.Poppema S, Potters M, Visser L.et al Immune escape mechanisms in Hodgkin's disease. Ann Oncol 19989(Suppl 5)S21–S24. [DOI] [PubMed] [Google Scholar]
- 126.Kapp U, Yeh W ‐ C, Patterson B.et al Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed‐Sternberg cells. J Exp Med 19991891939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed‐Sternberg cells. A possible explanation for the characteristic T‐cell infiltrate in Hodgkin's lymphoma. Am J Pathol 19991541685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oudejans J J, Jiwa N M, Kummer J A.et al Analysis of major histocompatibility complex class I expression on Reed‐Sternberg cells in relation to the cytotoxic T‐cell response in Epstein‐Barr virus‐positive and ‐negative Hodgkin's disease. Blood 1996873844–3851. [PubMed] [Google Scholar]
- 129.Murray P G, Constandinou C M, Crocker J.et al Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein‐Barr virus‐positive Hodgkin's disease. Blood 1998922477–2483. [PubMed] [Google Scholar]
- 130.Oudejans J J, Jiwa N M, Kummer J A.et al Activated cytotoxic T cells as prognostic marker in Hodgkin's disease Blood1997891376–1382. [PubMed] [Google Scholar]
- 131.Lee S P, Constandinou C M, Thomas W A.et al Antigen presenting phenotype of Hodgkin Reed‐Sternberg cells: analysis of the HLA class I processing pathway and the effects of interleukin‐10 on Epstein‐Barr virus‐specific cytotoxic T‐cell recognition. Blood 1998921020–1030. [PubMed] [Google Scholar]
- 132.Sing A P, Ambinder R F, Hong D J.et al Isolation of Epstein‐Barr virus (EBV)‐specific cytotoxic T lymphocytes that lyse Reed‐Sternberg cells: implications for immune‐mediated therapy of EBV+ Hodgkin's disease. Blood 1997891978–1986. [PubMed] [Google Scholar]
- 133.Roskrow M A, Suzuki N, Gan Y j.et al Epstein‐Barr virus (EBV)‐specific cytotoxic T lymphocytes for the treatment of patients with EBV‐positive relapsed Hodgkin's disease. Blood 1998912925–2934. [PubMed] [Google Scholar]
- 134.Smith C, Cooper L, Burgess M.et al Functional reversion of antigen‐specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J Immunol 20061774897–4906. [DOI] [PubMed] [Google Scholar]
- 135.Su Z, Peluso M V, Raffegerst S H.et al The generation of LMP2a‐specific cytotoxic T lymphocytes for the treatment of patients with Epstein‐Barr virus‐positive Hodgkin disease. Eur J Immunol 200131947–958. [DOI] [PubMed] [Google Scholar]
- 136.Duraiswamy J, Sherritt M, Thomson S.et al Therapeutic LMP1 polyepitope vaccine for EBV‐associated Hodgkin disease and nasopharyngeal carcinoma. Blood 20031013150–3156. [DOI] [PubMed] [Google Scholar]
- 137.Wagner H J, Bollard C M, Vigouroux S.et al A strategy for treatment of Epstein‐Barr virus‐positive Hodgkin's disease by targeting interleukin 12 to the tumor environment using tumor antigen‐specific T cells. Cancer Gene Ther 20041181–91. [DOI] [PubMed] [Google Scholar]
- 138.Bollard C M, Rossig C, Calonge M J.et al Adapting a transforming growth factor beta‐related tumor protection strategy to enhance antitumor immunity. Blood 2002993179–3187. [DOI] [PubMed] [Google Scholar]
- 139.Gandhi M K, Lambley E, Duraiswamy J.et al Expression of LAG‐3 by tumor‐infiltrating lymphocytes is coincident with the suppression of latent membrane antigen‐specific CD8+ T‐cell function in Hodgkin lymphoma patients. Blood 20061082280–2289. [DOI] [PubMed] [Google Scholar]