Abstract
Apocrine metaplasia is a very common finding in the female breast after the age of 25. It is so common that many people regard it as a normal component of the breast. This, however, is only really the case in apocrine sweat glands of the axilla and in the peri‐areolar apocrine glands. The apocrine cell does, however, contribute to a number of different breast lesions, some of which are very taxing diagnostically; apocrine variants of both in‐situ and invasive cancer are encountered. This review considers the common apocrine metaplastic lesions seen in fibrocystic change as well as apocrine adenoma, apocrine change within sclerosing adenosis, atypical apocrine lesions and apocrine malignancies.
Keywords: apocrine, metaplasia, breast, atypical, adenosis, apocrine carcinoma
Apocrine cells appear columnar, cuboidal or flattened, depending entirely on their location within acini or lining a cyst (fig 1). On H&E staining, two distinct types of apocrine cells are evident, although some may show intermediate features. In the first type, the cytoplasm is granular and strongly eosinophilic; a supranuclear vacuole, which may contain yellow brown pigment rich in iron or haemosiderin, is frequently present. The apical portion of the cell contains coarse birefringent granules. The nuclei are generally globoid and are usually pale with one or two prominent nucleoli, but they may be hyperchromatic as is usually seen in flattened apocrine epithelium in tension cysts, when nucleoli are not visible. The second type has a cytoplasm that is distinctly foamy with small vacuoles that may coalesce and show lipofuscin pigment in their cytoplasm.1 The nuclei are usually central and show the same features as the first type.
Figure 1 Apocrine metaplasia within fibrocystic change. H&E.
Apocrine cells stain with periodic acid‐Schiff after diastase digestion and are immunoreactive for epithelial membrane antigen (EMA) and cytokeratins 8 and 18. Normal apocrine cells do not express oestrogen (ER) or progesterone receptors (PR) but are positive for androgen receptors (AR).2,3 They also stain with three proteins found in cyst fluid, called gross cystic disease fluid protein (GCDFP) 15, 24 and 44.4 GCDFP15 is identical to prolactin inducible protein, GCDFP24 is apolipoprotein D and GCDFP44 is zinc α2 glycoprotein.
Metaplastic apocrine cells within the breast are similar to normal axillary, areolar and perineal apocrine cells, having apocrine secretions and identical staining reactions. One of the reasons, however, that these are often not regarded as normal components is that examples of gradual metaplastic change from the normal cuboidal epithelium can be seen and cells with intermediate features can also be discerned on careful examination, suggesting that these are true metaplasia (fig 2). This view is, however, often disputed. The mechanism for the development of apocrine metaplasia from normal cuboidal epithelium is not well characterised, but it is a pathognomic component of fibrocystic change and in type I breast cysts.5
Figure 2 A portion of a breast acinus showing metaplastic apocrine change with a transition between normal and metaplastic epithelium. H&E.
Apocrine lesions in the female breast
Apocrine change in the breast is seen in a broad spectrum of lesions ranging from microscopic cysts to invasive apocrine carcinoma. Not only are some of these lesions difficult to categorise, but also there is controversy regarding their relative risk of subsequent carcinoma development. Fibrocystic change, fibroadenomas, hamartomas, papillomas, sclerosing adenosis and apocrine adenoma are all benign breast lesions that can show apocrine change as one of their histological features.
Apocrine lesions can be divided into simple and papillary apocrine changes, those of uncertain significance, those found in association with other lesions, apocrine ductal carcinoma in‐situ and invasive apocrine carcinoma. In addition to carcinomas with obvious apocrine differentiation, other carcinomas have some apocrine features and it may be that a significant number of breast tumours arise from apocrine epithelium or that a significant number can express some apocrine features.
Apocrine metaplasia in fibrocystic change
Fibrocystic change is an extremely common finding, which is evident in about 50% of women of reproductive age.6 Formation of cysts is one of the basic morphological criteria of this disease. These cysts can be microscopic or grossly visible. Miller et al,5 categorised cysts into two types based on their electrolyte content. Type I cysts have a high concentration of potassium and low concentrations of sodium and chloride (K:Na ratio >1.5). High concentrations of androgen and oestrogen conjugates and epidermal growth factor are also present in this type. Type II cysts have high concentrations of sodium and chloride and low concentrations of potassium (K:Na ratio <1.5). Lower concentrations of sex hormones and epidermal growth factor are also a feature of type II cysts. The cyst lining was found to be closely related to the content, where type I cysts are usually lined by apocrine cells and type II cysts are usually lined by flattened epithelium. There was initial evidence to suggest that type I cysts had a higher likelihood of recurrence than type II cysts,7,8 but this has not been reproduced in studies with larger numbers of patients and longer follow‐up.9 Women with palpable cysts who have undergone aspiration, have been reported to have a slightly increased risk of subsequent carcinoma development.10,11 However, two of the largest studies with long term follow‐up showed conflicting results. The current consensus is that gross cysts are not associated with any significant increased risk of subsequent carcinoma development, therefore these women do not require any further follow‐up than would be offered routinely.12,13 The study by Tsung et al14 revealed that apocrine cells were present in both type I and type II cysts and that cyst type could not predict the likelihood of subsequent carcinoma development.
The incidence of apocrine cysts found in normal breasts obtained from autopsies is said to be as high as 85%.15 This prompted Eusebi et al16 to conclude that apocrine features should be considered a normal change in breast epithelium rather than a true disease. Wellings and Alpers17 examined the frequency and age distribution of apocrine cysts in normal women versus women who developed cancer in the same or contralateral breast. They reported that apocrine cysts were more common in cancer‐associated breasts (83%) than in normal breasts (52%).
Also, the average number of foci with apocrine cysts was higher in cancer‐associated breasts. They concluded that apocrine metaplasia (APM) could be a manifestation of epithelial unrest. Their conclusion led Haagensen18 to suggest that APM of the breast is either a precursor of malignant transformation in itself or that the metaplasia is a reflection of an underlying stimulus that renders the breast more susceptible to neoplasia.
Microscopic cysts lined by a single layer of apocrine epithelium are non‐proliferative lesions that are not associated with any increased risk of subsequent carcinoma development.12,19
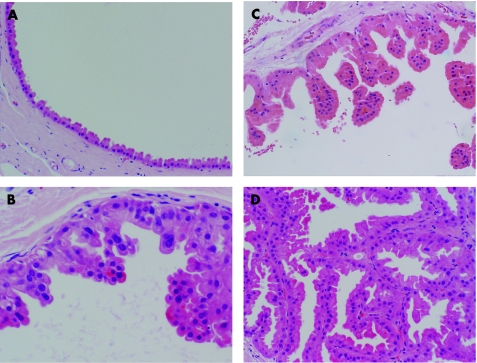
In 1996, Page et al20 stratified apocrine cysts lined by papillary apocrine epithelium into categories of increasing complexity using a combination of histological and cytological pattern rules. In this study, papillary apocrine change (PAC) was categorised into three forms: simple PAC, complex PAC and highly complex PAC (fig 3). Simple PAC can be identified when the epithelium lining an apocrine cyst is focally 3 or more cells thick, with the resulting mounds of cells showing no tendency to touch one another. These clumps of cells are broader at the base than at the tip.
Figure 3 Increasing degrees of complexity of apocrine metaplasia. (A) Non‐papillary apocrine metaplasia. (B) Simple papillary apocrine metaplasia. (C) Complex papillary apocrine metaplasia. (D) Highly complex papillary apocrine metaplasia. H&E.
In complex PAC, the papillae were taller, more attenuated and showed a tendency to touch each other within the lumen. Highly complex PACs were identified by the greatly elongated papillae, usually 2 or 3 cells in width, forming narrow arcades of apocrine cells intertwined with other papillations. This last category was quite uncommon, being only 1% of all reviewed biopsy specimens. The nuclear features were the same as those seen in unremarkable apocrine change. The authors reported that although there was a slight increased risk of subsequent carcinoma development in association with PAC, most of the increased risk was related to the presence of atypical hyperplasia of ductal (non‐apocrine) type (ADH). The relative risk was only 1.2 after women with ADH were excluded. Women with highly complex patterns of PAC without ADH did, however, experience a relative risk of 2.4, but due to the small number of cases, this did not achieve statistical significance. Apocrine cells do show a degree of nuclear variability, even in simple epithelium. This is partly a product of cross cutting of a large nucleus and partly an intrinsic property of apocrine epithelium. This phenomenon is recognised by most cytologists, especially in cyst fluids; even moderate degrees of nuclear variation and atypia are ignored unless there is necrosis or significant mitotic activity.
Apocrine adenoma
These lesions are extremely rare and the number of cases reported is not sufficient to determine the level of risk associated with these. They are unusual adenomas composed entirely of apocrine cells and are generally accepted to be benign. Pure breast adenomas with apocrine differentiation were described by Hertel et al in 1976.21 The criteria require the lesion to be homogenous throughout; to be sharply demarcated from the surrounding breast tissue; to have only epithelial proliferative elements; and to have a minimal, supportive stromal component.
Apocrine lesions of uncertain significance
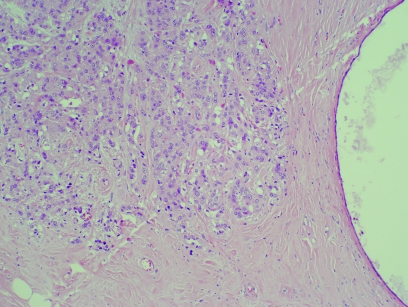
Apocrine change in sclerosing adenosis (apocrine adenosis)
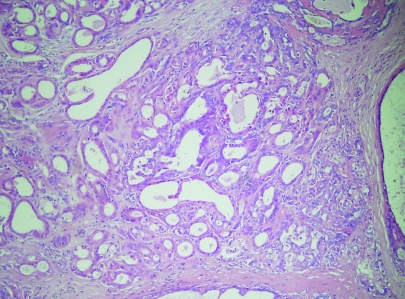
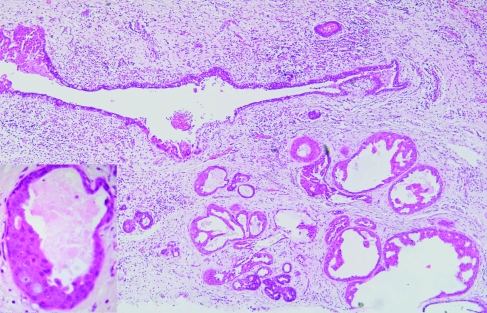
This has been called apocrine adenosis in the past, but this term has also been used for a completely different condition.22 It is therefore better to use the more descriptive term, “apocrine change in sclerosing adenosis” to avoid confusion with the lesion described in association with adenomyoepithelioma. This is a rare lesion, defined as the presence of apocrine cytology in a recognisable lobular unit associated with sclerosing adenosis (fig 4). It can show marked nuclear pleomorphism and, when this amounts to a threefold variation in nuclear size, has been designated “atypical apocrine adenosis” (fig 5).23 These workers ascribed a relative risk of 5.5 to this lesion, but only in the postmenopausal setting. The number of cases, however, was low and therefore the true risk is still somewhat uncertain. This lesion is sometimes also seen in association with radial scars and can be extremely difficult to distinguish from cancerisation of lobules by apocrine ductal carcinoma in‐situ (DCIS). Helpful features are the presence of necrosis or mitotic activity, which are not generally seen in apocrine change within sclerosing adenosis.
Figure 4 Apocrine change within sclerosing adenosis. H&E.
Figure 5 Atypical apocrine change within sclerosing adenosis showing marked variation in nuclear size. H&E.
Atypical apocrine hyperplasia
This condition is an intra‐ductal or lobular lesion composed of recognisable apocrine epithelium but with architectural patterns and/or cytological atypia, which are not normally seen in papillary apocrine metaplasia (fig 6). The architectural changes seen in this condition that are not generally found in papillary apocrine metaplasia include Roman bridges, cribriform patterns with round holes and multiple micropapillary fronds without connective tissue cores.24 Nuclear changes having a threefold variation in nuclear size and prominent nucleoli sometimes eosinophilic, are cytological changes, which are not often seen in standard papillary apocrine change. Atypical apocrine hyperplasia is currently insufficiently studied and its relationship to apocrine ductal carcinoma in‐situ is unclear but it is likely that this represents a precursor lesion. One of the problems of studying this lesion is that it has marked similarities to apocrine ductal carcinoma in‐situ and the criteria to distinguish these two lesions are not clearly defined. Features that can be used to attempt to distinguish these conditions are similar to those seen in atypical apocrine change in that necrosis and mitotic activity are not generally seen in atypical apocrine hyperplasia. Furthermore, established apocrine ductal carcinoma in‐situ usually has periductal or peri‐acinar fibrosis around the ducts or acini containing the in‐situ proliferation and usually shows a periductal or peri‐acinar lymphocytic infiltrate. These features are generally not seen in atypical apocrine hyperplasia.
Figure 6 Atypical apocrine hyperplasia. This example does have some lymphocytic infiltrate but does not have sufficient features to warrant designation as apocrine ductal carcinoma in‐situ. Inset: High power showing nuclear pleomorphism. H&E.
Apocrine change within other lesions
Radial scars
As indicated above, apocrine change and atypical apocrine changes can be a feature of radial scars and there may be marked distortion of the architecture due to the elastosis associated with these. Assessment of the apocrine lesion itself should be performed in isolation from the association with the radial scar, but bearing in mind that there may be fibrosis and lymphocytic infiltration secondary to the process causing the radial scar; this may not be indicative of periductal fibrosis and inflammation associated with apocrine ductal carcinoma in‐situ.
Papillomas
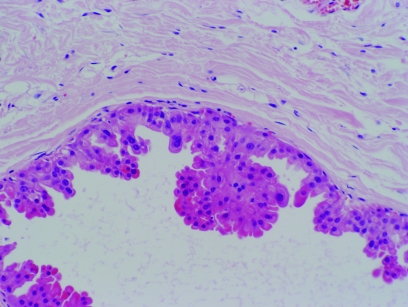
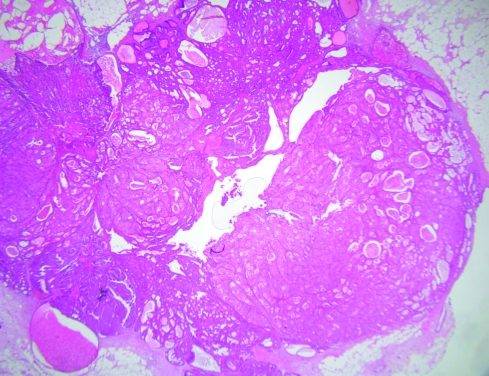
Apocrine change is frequently associated with benign papillary lesions. This apocrine change can have rather complex architectural appearances and can also be very solid, with marked nuclear atypia (fige 7). This often leads to the differential diagnosis between apocrine ductal carcinoma in‐situ arising in a papilloma and atypical apocrine change within a papilloma (atypical papilloma). These papillary lesions are generally found in lactiferous sinuses; in general, the authors favour a diagnosis of atypical papilloma unless there is obvious apocrine ductal carcinoma in‐situ affecting the ducts outside the papillary lesion. The reason for this is that invasive apocrine carcinomas around major nipple ducts associated with papillary lesions are vanishingly rare and the authors do not recall ever seeing this association despite an extensive referral practice. To our knowledge, this is also not well represented in the literature. Other terms for these papillary lesions when there is fibrosis obliterating much of the papillary nature, include sclerosing duct papilloma and ductal adenoma.
Figure 7 Solid apocrine hyperplasia within a papilloma. H&E.
Fibroadenomas and phyllodes tumours
Apocrine change within fibroadenoma is seen in approximately 10% of fibroadenomas.25 Fibroadenomas having cysts over 3 mm, sclerosing adenosis, epithelial hyperplasia or papillary apocrine metaplasia were described as complex fibroadenomas by Dupont et al.26 These were associated with an increased relative risk of 3.1 times in this study. The apocrine metaplasia is not usually atypical and indicates the hamartomatous nature of these proliferations. Apocrine metaplasia may also be seen in hamartomas but is not a feature of phyllodes tumours. This is because phyllodes tumours are clonal; metaplastic changes would neither be expected, nor appear to occur, in this neoplastic process.
Malignant apocrine lesions
Apocrine ductal carcinoma in‐situ
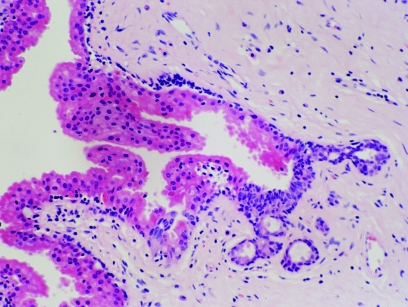
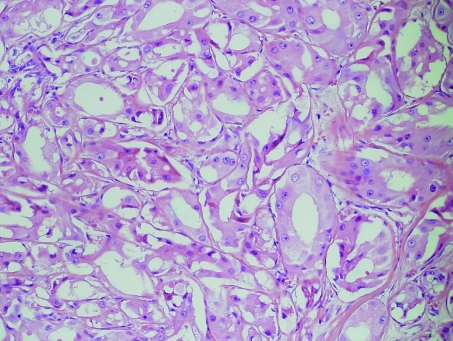
Apocrine differentiation in DCIS is one of the most under‐recognised changes in breast pathology. This phenomenon is only occasionally alluded to in the literature and whenever this lesion is discussed, a cautious approach is usually advised.24 Apocrine DCIS characterised by extensive proliferation, marked nuclear pleomorphism, multiple prominent nucleoli and comedo‐type necrosis, represents the least diagnostic difficulty (fig 8).
Figure 8 High grade apocrine ductal carcinoma in‐situ. H&E.
Although apocrine DCIS can also be diagnosed in cases with lesser degrees of nuclear pleomorphism, the diagnostic challenge in such cases is the accurate categorisation of the nuclear grade.27 The participants of the Consensus Conference on the classification of ductal carcinoma in‐situ, in 1997,28 recognised apocrine DCIS as a special variant, but no recommendations on sub‐classification were made.
More recently, Leal et al29 attempted to define criteria for low‐grade apocrine DCIS. They stratified their cases into low, intermediate and high histological grade according to nuclear grade and the presence of comedo‐type necrosis. Nuclear grading was assigned to one of three grades according to nuclear size, pleomorphism and the characteristics of the nucleoli compared with the nuclei of benign apocrine cells. Nuclear size was defined as small (1× to 2×), intermediate (3× to 4×) and large (5× or >5× the median size of the nuclei of normal apocrine cells).
Grade 1 nuclei were characterised by little pleomorphism, small or intermediate size and usually a single prominent nucleolus. Grade 2 nuclei showed moderate pleomorphism, small or intermediate size and some multiple nucleoli. Occasional large nuclei and/or multinucleated cells, with definite apocrine features and only mild to moderate pleomorphism, could be observed in nuclear grade 2 lesions and were not considered sufficient to imply nuclear upgrading. Grade 3 nuclei were of intermediate or large size and showed marked pleomorphism with a coarse chromatin pattern, irregular nuclear contour and frequent multiple nucleoli. In their series, low‐grade apocrine DCIS was defined by the presence of grade 1 or 2 nuclei and no evidence of necrosis. High‐grade apocrine DCIS had grade 3 nuclei (showing multiple nucleoli and coarse chromatin) as well as extensive necrosis. Intermediate grade apocrine DCIS included those cases showing grade 1 or 2 nuclei with necrosis as well as cases with high‐grade nuclei and no necrosis. Striking tumour heterogeneity was evident, which led the authors to speculate that low‐grade apocrine DCIS may be a precursor of high‐grade lesions.
Some pathologists also advocate mitotic activity, periductal fibrosis and inflammation as a feature helpful in the diagnosis of apocrine DCIS.30
To conclude, apocrine DCIS should be graded, and because of the marked heterogeneity, the nuclear grading should be based on the foci showing the greatest degree of pleomorphism. Although apocrine DCIS is usually associated with intraluminal necrosis, the presence of necrosis is not necessary for the diagnosis of this lesion; however, caution should be taken to avoid the over‐diagnosis of a benign apocrine proliferative lesion.13,24 This is especially important as even “normal” apocrine metaplastic cells may show moderate degrees of pleomorphism.
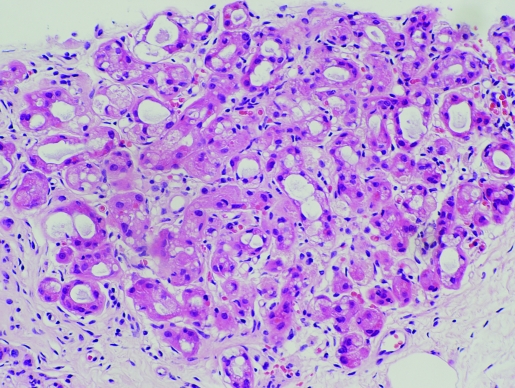
Invasive apocrine carcinoma
The incidence of pure apocrine carcinoma (fig 9) varies from <0.3% to 4%.25,31 This variability in incidence is likely to be a result of the lack of well‐defined diagnostic criteria. Rosen32 stipulates that the term should be reserved for neoplasms in which all or nearly all the epithelium shows apocrine cytological features. In contrast, focal apocrine differentiation is quite common and has been reported in up to 60% of carcinomas of no special type (NST), “ductal NST”.33
Figure 9 Invasive apocrine carcinoma. H&E.
When the apocrine phenotype is based on the immunoexpression of the 15 kDa glycoprotein of gross cystic disease fluid (GCDFP), the incidence is as high as 72%.34 Areas of apocrine differentiation have also been reported in special type cancers, including papillary and lobular carcinomas.35,36 The apocrine phenotype can be further corroborated by additional studies, including the presence of periodic acid‐Schiff positive cytoplasmic granules and the demonstration of empty vesicles and osmiophilic granules at the ultrastructural level.37,38
Grossly, these tumours are indistinguishable from other mammary carcinomas.32 Tumour size, presentation, incidence of lymph node positivity and grade do not differ significantly from NST tumours. In two reports that specify the laterality of the disease, the left side predominates, a fact that should be interpreted with caution because the total number of invasive cancers in these two reports is only 51. These authors also concluded that apocrine carcinoma is not clinically distinct from NST carcinoma.39,40 Only two cases of bilateral apocrine carcinoma have been reported, one synchronous and the other metachronous.41,42
Microscopically, apocrine carcinomas show the same architectural growth pattern as other NST mammary carcinomas, differing only in their cytological appearance. Invasive carcinomas showing prominent apocrine change are characterised by a distinctive immunohistochemical profile, being ER−/PR−/AR+.43,44 In a recent series of a 145 carcinomas, 60% of NST invasive carcinomas were immunopositive for AR. However, in the 12 cases showing prominent apocrine differentiation, 7 (58%) were ER−/PR−/AR+. This study indicates that AR positivity may be common to all invasive carcinomas rather than being restricted to apocrine carcinoma.45
The above studies suggest that there is not enough evidence in the literature to recognise the apocrine phenotype as a special type carcinoma. However, a recent study by Japaze et al46 identifies a subtype of invasive apocrine carcinoma, namely, pure invasive apocrine carcinoma. According to their findings, pure invasive apocrine carcinoma may represent a distinct clinicopathological entity with a less aggressive behaviour than ductal carcinoma NST and might be regarded as an independent prognostic factor in early breast cancer. According to these authors, pure invasive apocrine carcinoma should contain large cells with abundant eosinophilic cytoplasm, usually granular, in cells with a nucleus:cytoplasm ratio of 1:2 or more. The cells should have round and/or pleomorphic, large vesicular nuclei similar to those in apocrine metaplasia, and sharply defined borders or linear and well‐defined cell margins. These features should be present in at least 75% of the microscopic fields studied. Other features which were often seen were prominent nucleoli in a high percentage of the fields (more than 50%) and apical convexity (cytoplasmic snouting) of the cytoplasm where there were luminal spaces.
Cases with clear cells of any type comprising any proportion of the cell total and specific types of carcinomas (tubular, lobular, mucinous, invasive micropapillary and medullary) in association with invasive apocrine carcinoma, exclude these tumours from the category of pure invasive apocrine carcinoma.
Apocrine cells in cytological preparations
Apocrine cells are often seen in association with macrophages and benign duct epithelial cells in smears from fibrocystic change and also in smears from breast cyst fluids. Benign apocrine cells may occasionally be rather worrisome in breast cysts, especially if these are inflamed. Although clear cyst fluids need not be examined, Tsung et al14 recommended that all cloudy or turbid cyst fluid should be cytologically examined, based on their finding of malignancy in occasional samples with turbid fluid; furthermore, the various guidelines on cytology suggest that cloudy or bloodstained cyst fluids should be examined.47
Atypical apocrine cells from fibrocystic change, and especially apocrine change in sclerosing adenosis, may be extremely difficult to distinguish from apocrine carcinoma. If there are mitoses or if necrosis is present, carcinoma is the most likely diagnosis; but if these are absent, it is wise to be cautious. In cases of apocrine change within sclerosing adenosis, two cell types may be visible in the groups; in this instance a suspicious (C4) result is most appropriate.48
Apocrine lesions in core biopsy specimens
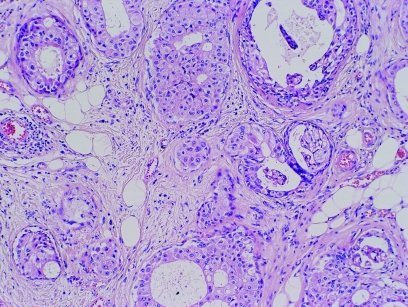
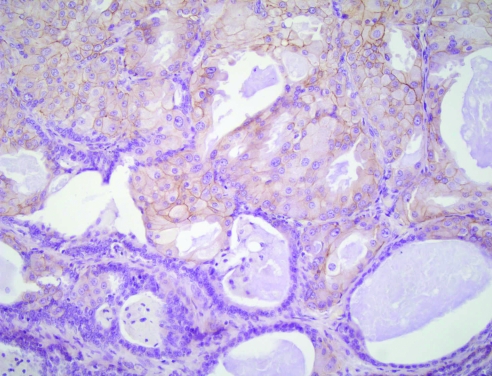
Apocrine lesions can cause difficulty in core biopsy specimens, especially when there is a small area of atypical apocrine adenosis (fig 10) or atypical apocrine hyperplasia. In the case of atypical apocrine adenosis, the problem is distinguishing this from cancerisation of lobules by apocrine type ductal carcinoma in‐situ. As in cytological preparations, the presence of necrosis or mitotic activity is indicative of the latter, but in cases where these are not present, it may be difficult to be certain. Her‐2 staining may help as, although atypical apocrine proliferations sometimes show weak positive membrane staining (fig 11), very strong membrane staining or amplification on fluorescent in situ hybridisation testing is more likely to be seen in carcinoma.49 Atypical apocrine hyperplasia in cores may also be indicative of more established apocrine ductal carcinoma in‐situ nearby. In both these conditions further levels are mandatory to exclude more diagnostic areas deeper in the core and, if not, a designation of “lesion of uncertain biological behaviour” (B3) is recommended. Excision biopsy or vacuum biopsy excision is advised for these unusual lesions.
Figure 10 Apocrine change within sclerosing adenosis in a core biopsy specimen. H&E.
Figure 11 Her‐2 staining of solid apocrine hyperplasia within a papilloma, showing some membrane staining of the apocrine cells (same case as fig 7). H&E.
Immunohistochemical profile of apocrine cells
Apocrine cells show immunoreactivity for epithelial membrane antigen and cytokeratins 8 and 18, but these are not specific for the apocrine cell type. Alpha‐1 antitrypsin and lysozyme are occasionally positive.16 Characteristically, the cells are also known to be bcl‐2 and S‐100 negative.50 ER and PR are also negative while AR is consistently positive.2,3 Shim et al51 investigated the importance of androgens and their receptors inhibin and activin and concluded that AR and activin A may be implicated in apocrine morphogenesis, but not in tumour progression. It is interesting that type I breast cysts have a high androgen content and it may be that there is a stimulatory feedback loop within some of these cysts producing proliferation and hence papillary apocrine changes.
Gross cystic disease fluid proteins 15, 24 and 44 are major components of aspirated cyst fluid and are characteristic of apocrine cells. An antibody against GCDFP‐15 stains the cytoplasm of apocrine cells diffusely, predominating in the apical region where the periodic acid‐Schiff positive granules are located. When the cytoplasm is foamy, the stain tends to be diffuse throughout the cytoplasm.52 The protein was reported to be absent in other tissues, with the exception of some acini of normal salivary glands and apocrine glands in other sites. This was attributed to the fact that salivary glands share common functions with apocrine glands.37,53
GCDFP‐24 or apolipoprotein‐D antibodies show these proteins to be localised to apocrine epithelium within axillary skin. There is no localisation to eccrine glands, sebaceous glands or hair follicles.4 Antibodies against GCDFP‐44 show the protein to be localised in apocrine glands and metaplastic apocrine epithelium of the breast. Parotid gland and some eccrine glands of the skin from the palm and sole of the foot are also positively stained.4,54
Selim et al55 reported the presence of all three proteins in all examined cases of apocrine metaplasia, but only 63.6%, 54.5% and 63.6% of their cases of apocrine change within sclerosing adenosis were positive for GCDFP‐15, GCDFP‐24 and GCDFP‐44, respectively. The authors also reported that in 9 cases of morphologically apocrine DCIS, only 77.8%, 55.6% and 66.7% of cases were positive for GCDFP‐15, GCDFP‐24 and GCDFP‐44, respectively. In their 57 cases of non‐apocrine DCIS, 42.1% were positive for GCDFP‐15, 35.1% were positive for GCDFP‐24 and 36.6% were positive for GCDFP‐44.
Based on these findings the authors suggested the possibility that, as some apocrine lesions progress from putative precursor lesions, “apocrine change in sclerosing adenosis” to DCIS, they tend to lose one or more of their apocrine proteins, and that some cases of DCIS could be derived from apocrine cells that have lost some of these proteins or have structurally abnormal proteins that could no longer be detected by the specific antibodies. The authors also indicated that there was no significant association between the degree of differentiation of DCIS and the degree of expression of these markers.
Recent proteome expression profiling studies of breast apocrine macrocysts, normal breast tissue, and breast tumours have identified specific apocrine biomarkers (15‐hydroxyprostaglandin dehydrogenase and hydroxymethylglutaryl coenzyme A reductase) present in early and advanced apocrine lesions. These biomarkers, in combination with proteins found to be characteristically up‐regulated in pure apocrine carcinomas (psoriasin, S100A9, and p53), provide a protein expression signature distinctive for benign apocrine metaplasia and apocrine cystic lesions.56
Tokes et al57 studied some of the proteins involved in the formation of tight junctions in epithelial cells. The authors studied the expression of CLDN1 and CLDN2 (two members of the claudin family which are proteins involved in the formation of tight junctions) and stated that CLDN1 was present in eight out of the nine areas of APM studied as well as in normal breast epithelium. The protein was down‐regulated in cases of invasive breast carcinoma, indicating that the protein might play a role in invasion and metastasis. On the other hand, CLDN4 was consistently absent in all areas of APM as well as in samples of special type carcinomas (mucinous, tubular and papillary), where the protein was either down‐regulated or absent, indicating that CLDN4 might play a role in cellular differentiation.
Response to therapy and predictive profile
Earlier studies in the literature have addressed the subject of clinical significance of the apocrine phenotype. Comparison of invasive apocrine carcinomas with NST tumours matched for diagnostic parameters (e.g., stage) and time of diagnosis, revealed no statistical difference in estimated recurrence‐free survival or overall survival.39,40,58 However, the study by Japaze et al,46 referred to above, suggests that pure invasive apocrine carcinoma may represent a distinct clinicopathological entity with a less aggressive behaviour than IDC‐NOS and might be regarded as an independent prognostic factor in early breast cancer.
Several other studies investigating the immunohistochemical profile of apocrine lesions have shown that the apocrine phenotype is mostly associated with a characteristic profile (AR+/ER−/PR−/Bcl‐2‐ve). These findings prompt the possibility that these lesions might benefit from a different therapeutic regime than that suggested for NST carcinomas.2,3,59
Take‐home messages
Apocrine change can be seen in both benign and malignant lesions of the breast.
Pathologists need to be aware of atypical apocrine lesions and atypical apocrine cells in cysts to avoid over‐diagnosis of malignancy.
Apocrine ductal carcinoma in‐situ can be difficult to grade.
The concept that apocrine morphology may be associated with significant biological differences is further highlighted by recent molecular studies. Kasami et al60 reported that androgen receptor CAG repeat lengths were longer in DCIS than in fibroadenomas or invasive carcinomas of the female breast, raising the possibility that longer CAG repeats could prevent the development of invasive disease. The authors also report that CAG repeats were longest in DCIS cases with apocrine features. Given these facts, future study of CAG repeat lengths in various apocrine lesions (metaplasia, apocrine change in sclerosing adenosis, apocrine DCIS and invasive apocrine carcinoma) might prove to be of benefit towards designing genotype based regimens for prevention and treatment of these lesions.
Jones et al61 showed that invasive apocrine carcinomas have areas of loss and gain in common with invasive carcinomas of no special type, but also harbour alterations not previously reported as playing a significant role in breast carcinogenesis. These include losses at 2p and 9q and gains at 2q, 3p and 13q.
Farmer et al,62 using gene expression microarrays, identified a group of tumours which were mainly characterised by apocrine features on histological examination, AR positive and ER negative hormone profiles and c‐erbB2 amplification. These tumours were referred to as the “molecular apocrine group” and were found to represent 8–14% of the tumours studied by this technique. The clinical significance of these findings remains to be seen in future studies.
Abbreviations
ADH - atypical ductal hyperplasia
APM - apocrine metaplasia
AR - androgen receptor
DCIS - ductal carcinoma in‐situ
ER - oestrogen receptor
GCDFP - gross cystic disease fluid protein
NST - no special type
PAC - papillary apocrine change
PR - progesterone receptor
Footnotes
Competing interests: None declared.
References
- 1.Bussolati G, Cattani M, Gugliotta P.et al Morphologic and functional aspects of apocrine metaplasia in dysplastic and neoplastic breast tissue. Ann NY Acad Sci 1986464262–274. [DOI] [PubMed] [Google Scholar]
- 2.Tavassoli F, Purcell C, Bratthauer G.et al Androgen receptor expression along with loss of Bcl‐2, ER, and PR expression in benign and malignant apocrine lesions of the breast: Implications of therapy. Breast J 19962261–269. [Google Scholar]
- 3.Selim A, Wells C. Immunohistochemical localisation of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to estrogen and progesterone receptors. J Clin Pathol 199952838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazoujian G, Haagensen D., Jr The immunopathology of gross cystic disease fluid proteins. Ann NY Acad Sci 1990586188–197. [DOI] [PubMed] [Google Scholar]
- 5.Miller W, Scott W, Phil M.et al Using biological measurements, can patients with benign breast disease who are at high risk of breast cancer be identified. Cancer Detect Prev 199216(Suppl)13–20. [PubMed] [Google Scholar]
- 6.Devitt J. Clinical benign disorders of the breast and carcinoma of the breast. Surg Gynecol Obstet 1981152437–440. [PubMed] [Google Scholar]
- 7.Bruzzi P, Dogliotti L, Naldoni C.et al Cohort study of association of risk of breast cancer with cyst type in women with gross cystic disease of the breast. BMJ 1997314925–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon J, Lumsden A, Miller W. The relationship of cyst type to risk factors for breast cancer in patients with breast cystic disease. Eur J Cancer 1985211047–1050. [DOI] [PubMed] [Google Scholar]
- 9.Dixon J, McDonald C, Miller W. Risk of breast cancer in women with palpable breast cysts: a prospective study. Lancet 19993531742–1745. [DOI] [PubMed] [Google Scholar]
- 10.Bundred N J, West R, Dowd J.et al Is there an increased risk of breast cancer in women who have had a breast cyst aspirated? Br J Cancer 199164953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devitt J, To T, Miller A. Risk of breast cancer in women with breast cysts. Can Med Assoc J 199214745–49. [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgibbons P, Henson D, Hutter R. Benign breast changes and the risk of subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med 19981221053–1055. [PubMed] [Google Scholar]
- 13.O'Malley F, Bane A. The spectrum of apocrine lesions of the breast. Adv Anat Pathol 2004111–9. [DOI] [PubMed] [Google Scholar]
- 14.Tsung J, Wang T, Wang S.et al Cytological and biochemical studies of breast cyst fluid. Breast 20051437–41. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen M, Thomsen J, Primdhal S.et al Breast cancer atypia among young and middle aged women: a study of 110 medicolegal autopsies. Br J Cancer 198756814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eusebi V, Damiani S, Losi L.et al Apocrine differentiation of breast epithelium. Adv Anat Pathol 19974139–155. [Google Scholar]
- 17.Wellings S, Alpers C. Apocrine cystic metaplasia: subgross pathology and prevalence in cancer‐associated versus random autopsy breasts. Hum Pathol 198718381–386. [DOI] [PubMed] [Google Scholar]
- 18.Haagensen D. Is cystic disease related to breast cancer. Am J Surg Pathol 199115687–694. [DOI] [PubMed] [Google Scholar]
- 19.Dupont W, Page D. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985312146–151. [DOI] [PubMed] [Google Scholar]
- 20.Page D, Dupont W, Jensen R. Papillary apocrine change of the breast: associations with atypical hyperplasia and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 1996529–32. [PubMed] [Google Scholar]
- 21.Hertel B, Zaloudek C, Kempson R. Breast adenomas. Cancer 1976372891–2905. [DOI] [PubMed] [Google Scholar]
- 22.Eusebi V, Cassadei G, Bussolati G.et al Adenomyoepithelioma of the breast with a distinct type of apocrine adenosis. Histopathology 198711305–315. [DOI] [PubMed] [Google Scholar]
- 23.Seidman J, Ashton M, Lefkowitz M. Atypical apocrine adenosis of the breast. A clinicopathologic study of 37 patients with 8.7 year follow up. Cancer 1996772529–2537. [DOI] [PubMed] [Google Scholar]
- 24.Tavassoli F, Norris H. Intraductal apocrine carcinoma: a clinicopathologic study of 37 cases. Mod Pathol 19947813–818. [PubMed] [Google Scholar]
- 25.Azzopardi J.Problems in breast pathology. Philadelphia, PA: WB Saunders, 197923–87.
- 26.Dupont W, Page D, Parl F.et al Long‐term risk of breast cancer in women with fibroadenoma. N Engl J Med 199433110–15. [DOI] [PubMed] [Google Scholar]
- 27.Scott M, Lagios M, Axelsson K.et al Ductal carcinoma in situ of the breast reproducibility of histological subtype analysis. Hum Pathol 199728967–973. [DOI] [PubMed] [Google Scholar]
- 28.The Consensus Conference Committee Consensus conference on the classification of ductal carcinoma in situ. Cancer 1997801798–1802. [DOI] [PubMed] [Google Scholar]
- 29.Leal C, Henrique R, Monteiro P.et al Apocrine ductal carcinoma in situ of the breast: histologic classification and expression of biologic markers. Hum Pathol 200132487–493. [DOI] [PubMed] [Google Scholar]
- 30.NHS BSP Guidelines for pathology reporting in breast disease. Publication 58, Version 2. Sheffield: NHS BSP, 2005
- 31.Mossler J, Barton T, Brinkhous A.et al Apocrine differentiation in human carcinoma. Cancer 1980462463–2471. [DOI] [PubMed] [Google Scholar]
- 32.Rosen P.Rosen's breast pathology, 2nd edn. Philadelphia, PA: Lippincott W and Wilkins 2001
- 33.Haagensen C.Diseases of the breast, 3rd edn. Philadelphia, PA: WB Saunders 1986
- 34.Wick M, Lillemoe T, Copland G.et al Gross cystic disease fluid protein 15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha lactalbumin. Hum Pathol 198920281–287. [DOI] [PubMed] [Google Scholar]
- 35.Papotti M, Eusebi V, Gugliotta P.et al Immunohistochemical analysis of benign and malignant papillary lesions of the breast. Am J Surg Pathol 19837451–461. [DOI] [PubMed] [Google Scholar]
- 36.Eusebi V, Magalhaes F, Azzopardi J. Pleomorphic lobular carcinoma of the breast: an aggressive tumour showing apocrine differentiation. Hum Pathol 199223655–662. [DOI] [PubMed] [Google Scholar]
- 37.Pagani A, Eusebi V, Bussolati G. Detection of PIP‐GCDFP15 gene expression in apocrine epithelium of the breast and salivary glands. Appl Immunohistochem 1994229–35. [Google Scholar]
- 38.Ahmed A.Atlas of the ultrastructure of the human breast diseases. Edinburgh: Churchill Livingstone, 197841–45.
- 39.D'Amore E, Terrier‐Lacombe M, Travagli J.et al Invasive apocrine carcinoma of the breast. A long term follow‐up study of 34 cases. Breast Cancer Res Treat 19881237–44. [DOI] [PubMed] [Google Scholar]
- 40.Abati A, Kimmel M, Rosen P. Apocrine mammary carcinomas. A clinicopathological study of 72 cases. Am J Clin Pathol 199094371–377. [DOI] [PubMed] [Google Scholar]
- 41.Frable W, Kay S. Carcinoma of the breast. Histologic and clinical features of apocrine tumours. Cancer 196821756–763. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt F, Soares R, Seruca R. Bilateral apocrine carcinoma of the breast. Molecular and immunocytochemical evidence for two independent primary tumours. Virchows Arch 1998433505–509. [DOI] [PubMed] [Google Scholar]
- 43.Gatalica Z. Immunohistochemical analysis of apocrine breast lesions. Consistent over‐expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract 1997193753–758. [DOI] [PubMed] [Google Scholar]
- 44.Sapp M, Malik A, Hanna W. Hormone receptor profile of apocrine lesions of the breast. Breast J 20039335–336. [DOI] [PubMed] [Google Scholar]
- 45.Moinfar F, Okcu M, Tsybrovskyy O.et al Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 200398703–711. [DOI] [PubMed] [Google Scholar]
- 46.Japaze H, Emina J, Diaz C.et al Pure invasive apocrine carcinoma of the breast: a new clinicopathological entity. Breast 2005143–10. [DOI] [PubMed] [Google Scholar]
- 47.European Working Group for Breast Screening Pathology (Chair: Wells CA) European guidelines for quality assurance in breast cancer screening and diagnosis, 4th edn. Chapter 6a 2006 ISBN 92‐79‐01258‐4
- 48.Makunura C N, Curling O M, Yeomans P.et al Apocrine adenosis within a radial scar: a case of false positive breast cytodiagnosis. Cytopathology 19945123–128. [DOI] [PubMed] [Google Scholar]
- 49.Selim A A, El‐Ayat G, Wells C A. C‐erbB2 oncoprotein expression, gene amplification and chromosome 17 aneusomy in apocrine adenosis of the breast: J Pathol 2000191138–142. [DOI] [PubMed] [Google Scholar]
- 50.Selim A A, El‐Ayat G, Wells C A. Expression of c‐erbB2, p53, Bcl‐2, Bax, c‐myc and Ki‐67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch 2002441449–455. [DOI] [PubMed] [Google Scholar]
- 51.Shim H S, Jung W H, Kim H.et al Expression of androgen receptors and inhibin/activin alpha and betaA subunits in breast apocrine lesions. APMIS 2006114352–358. [DOI] [PubMed] [Google Scholar]
- 52.Mazoujian G, Pinkus G S, Davis S.et al Immunohistochemistry of a gross cystic disease fluid protein (GCDFP‐15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol 1983110105–112. [PMC free article] [PubMed] [Google Scholar]
- 53.Haagensen D E, Jr, Dilley W G, Mazoujian G.et al Review of GCDFP‐15. An apocrine marker protein. Ann NY Acad Sci 1990586161–173. [DOI] [PubMed] [Google Scholar]
- 54.Bundred N J, Miller W R, Walker R A. An immunohistochemical study of the tissue distribution of the breast cyst fluid protein, zinc alpha 2 glycoprotein. Histopathology 198711603–610. [DOI] [PubMed] [Google Scholar]
- 55.Selim A, El‐Ayat G, Wells C. Immunohistochemical localisation of gross cystic fluid protein 15,24 and 44 in ductal carcinoma in situ of the breast; relationship to the degree of differentiation. Histopathology 200139198–202. [DOI] [PubMed] [Google Scholar]
- 56.Celis J E, Gromova I, Gromov P.et al Molecular pathology of breast apocrine carcinomas: a protein expression signature specific for benign apocrine metaplasia. FEBS Lett 20065802935–2944. [DOI] [PubMed] [Google Scholar]
- 57.Tokes A, Kulka J, Paku S.et al Claudin 1, 3 and 4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res 20057296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eusebi V, Millis R, Cattani M.et al Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol 1986123532–541. [PMC free article] [PubMed] [Google Scholar]
- 59.Selim A, El‐Ayat G, Wells C. Androgen receptor expression in ductal carcinoma in situ of the breast: relation to estrogen and progesterone receptors. J Clin Pathol 20025514–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasami M, Gobbi H, Dupont W.et al Androgen receptor CAG repeat lengths in ductal carcinoma in situ of breast, longest in apocrine variety. Breast 2000923–27. [DOI] [PubMed] [Google Scholar]
- 61.Jones C, Damiani S, Wells D.et al Molecular cytogenetic comparison of apocrine hyperplasia and apocrine carcinoma of the breast. Am J Pathol 2001158207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farmer P, Bonnefoi H, Becette V.et al Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 2005244660–4671. [DOI] [PubMed] [Google Scholar]