Abstract
Adult T‐cell leukaemia/lymphoma (ATLL) is a mature T‐cell neoplasm of post‐thymic lymphocytes aetiologically linked to the human T‐cell lymphotropic virus, HTLV‐I, and with a distinct geographical distribution. The disease manifests with leukaemia in greater than two thirds of patients, while the remaining patients have a lymphomatous form. According to the disease manifestations, various forms which differ in clinical course and prognosis have been recognised: acute, chronic, smouldering and lymphoma. Organomegaly, skin involvement, circulating atypical lymphocytes (“flower” cells) with a CD4+ CD25+ phenotype and hypercalcaemia are the most common disease features. The diagnosis should be based on a constellation of clinical features and laboratory investigations. The latter comprise: lymphocyte morphology, immunophenotype, histology of the tissues affected in the pure lymphoma forms and serology or DNA analysis for HTLV‐I. The differential diagnosis of ATLL includes other mature T‐cell neoplasms such as T‐cell prolymphocytic leukaemia (T‐PLL), Sézary syndrome (SS), peripheral T‐cell lymphomas and occasionally healthy carriers of the virus or Hodgkin disease. The clinical course is aggressive with a median survival of less than 12 months in the acute and lymphoma forms. Despite major advances in understanding the pathogenesis of the disease, management of these patients remains a challenge for clinicians as they do not respond or achieve only transient responses to therapies used in high‐grade lymphomas. The use of antiretroviral agents such as zidovudine in combination with interferon‐alpha, with or without concomitant chemotherapy, has shown activity in this disease with improvement in survival and response rate. Consolidation with high dose therapy and autologous or allogeneic stem‐cell transplantation should be considered in young patients.
Adult T‐cell leukaemia/lymphoma (ATLL) is a mature T‐lymphoid malignancy of post‐thymic pleomorphic activated T lymphocytes. This neoplasm is unique in its pathogenesis as it is aetiologically linked to the human T‐cell lymphotropic virus, HTLV‐I. HTLV‐I is detected in cells from virtually all cases of ATLL, with exceedingly rare exceptions. The disease is recognised under the WHO classification as a distinct entity.1
ATLL and HTLV‐I are prevalent in Japan, the Caribbean basin, and certain regions of South America and Africa, and in immigrants from these countries to other regions. It has also been recognised in patients originating from Iran and Central Europe. In the UK, ATLL is mainly, but not exclusively, seen in first or second generation immigrants of Afro‐Caribbean descent.2 The disease manifests in 75% of cases with leukaemia and in the remaining as a pure lymphomatous form.3 Shimoyama proposed criteria for the diagnosis of the various clinical forms in 19913; they are summarised in box 1. Usually the disease is disseminated, although lymphomas confined to the gut and central nervous system have been recognised.4,5,6 The outlook of ATLL is poor as most patients do not respond or have short‐lived partial responses to treatments used in other T‐cell lymphomas.
Box 1 Clinical forms of adult T‐cell leukaemia/lymphoma (ATLL)3
Acute
Leukaemic picture, organomegaly, high lactate dehydrogenase (LDH) and often hypercalcaemia
Chronic
Lymphocytosis >4×109/l with ATLL cells, skin, lung, liver or node involvement
Calcium levels normal, LDH normal or less that twice the upper normal limit
Smouldering
Skin and/or lung infiltrates
No other organ involvement
Normal lymphocyte count (1–5% ATLL cells), normal calcium and LDH
Lymphoma
Organomegaly
Less than 1% circulating leukaemic cells
High LDH and possible hypercalcaemia
This review will focus on the clinical manifestations, diagnostic features, treatment and outcome of patients with ATLL. Pathogenic and oncogenic mechanisms in HTLV‐I infection and ATLL are discussed elsewhere.7
Clinical features
ATLL affects almost exclusively adults and is extremely rare in children, although a few cases in childhood have been described.8 The median age is around the mid‐60s and there is no gender prevalence. Familial ATLL has been documented in Japan, the USA and England9,10; it is unknown whether a genetic predisposition plays a role in the development of the disease in such cases. In addition, ATLL may coexist or follow other HTLV‐I induced non‐neoplastic diseases such as tropical spastic paraparesis.11
Depending on the disease manifestations, ATLL is classified into several forms (box 1).3 The most common form of presentation is acute, which is seen in around 65% of patients. This is characterised by the presence of systemic symptoms, organomegaly, in particular lymphadenopathy, and a leukaemic picture. Hypercalcaemia with or without lytic bone lesions is present in half of these patients and it may develop during disease progression in another third. Skin lesions are seen in close to a half of these patients. The chronic form is characterised by lymphocytosis which may be stable for months or even years, skin manifestations, no organomegaly or small volume lymphadenopathy, absence of hypercalcaemia and normal or only slightly raised lactate dehydrogenase (LDH) (less than twice the upper normal limit value). Patients with smouldering ATLL are usually asymptomatic or manifest skin rashes that respond to topical steroids and/or lung infiltrates; unlike the chronic form, the white blood cell count is normal and there is no lymphocytosis and less than 3% atypical circulating lymphocytes. Less than a third of patients present with lymphoma with no evidence of blood involvement. There are several reports documenting disease progression to the acute form in patients with chronic and smouldering ATLL. Haemophagocytic syndrome as the first sign of transformation has been described in smouldering ATLL.12
In addition to the symptoms related to the neoplastic condition, patients with ATLL are immunocompromised and develop opportunistic infections that complicate the disease course and make its management more difficult. Infestation by Strongyloides stercoralis is frequent and may be severe and fatal. This association and the fact that a third of the patients with strongyloidiasis and positive serology for HTLV‐I have a clonal integration of the provirus in their lymphocytes, have led to the hypothesis that this parasite plays a role in the development of ATLL in healthy carriers.13
Haematopathological features
Peripheral blood
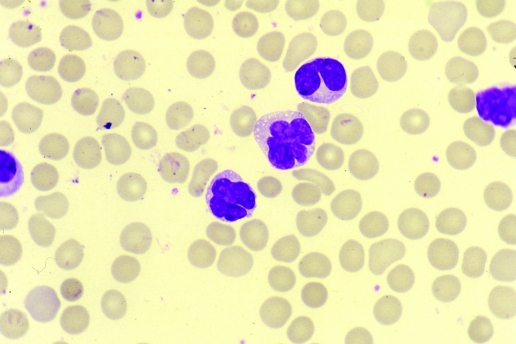
The incidence of anaemia and thrombocytopenia is variable. Neutrophilia and eosinophilia may be present. In the acute and chronic leukaemic forms, the white blood cell count is raised with circulating atypical cells. The cytological picture is pleomorphic, with the predominant cell being a medium size lymphocyte with condensed chromatin and a convoluted or polylobated nucleus, designated a “flower cell” (figs 1 and 2); nucleoli are not visible, and when identified are often small. The cytoplasm is medium or scanty and agranular with a variable degree of basophilia. A few blasts or immunoblast‐like cells with dispersed chromatin and prominent nucleolus may be present in the blood, but usually are more prominent in the lymphoid tissues involved.
Figure 1 Circulating lymphocytes in a patient with adult T‐cell leukaemia/lymphoma, showing a convoluted polylobated nucleus. May Grumwald Giemsa stain.
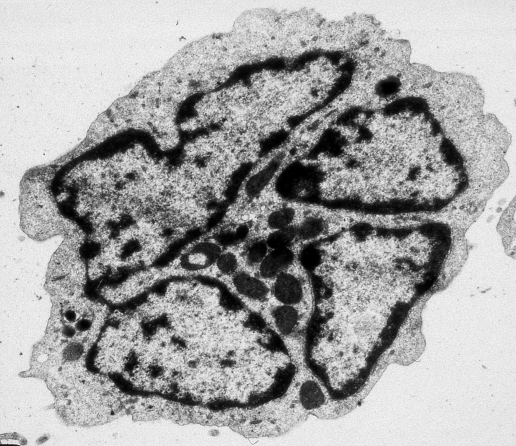
Figure 2 Electron micrograph of an adult T‐cell leukaemia/lymphoma cell, showing a nucleus integrated by different segments. Uranyl acetate and lead nitrate stain.
In the lymphomatous and smouldering forms of ATLL, the peripheral blood is not involved.
Bone marrow
The aspirate may show infiltration by lymphocytes with the same morphological features to those in the blood. The trephine biopsy may or may not show lymphoid infiltration, with the degree of involvement being variable. Even in the leukaemic forms of ATLL, the infiltration may be very subtle or patchy. The pattern of infiltration ranges from interstitial to diffuse.
A distinct feature in some cases with hypercalcaemia is the presence of increased osteoclasts with bone resorption.
Lymph node and other tissues
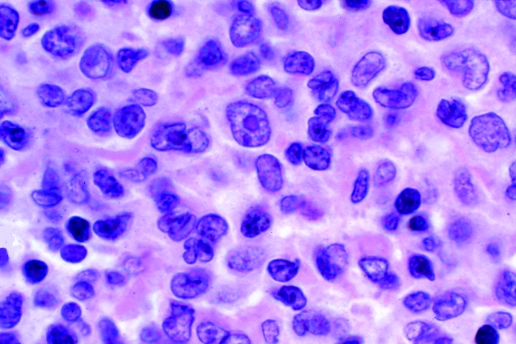
The infiltration in the lymph nodes is often diffuse, with the paracortical area expanded by an infiltrate of lymphocytes of various sizes with various nuclear shapes; as in the peripheral blood, pleomorphism is often a feature (fig 3); rarely, the infiltration is predominantly by small cells. There may be some immunoblast‐like and Reed–Sternberg‐like cells. The latter appear to be non‐neoplastic Epstein–Barr virus (EBV)‐infected cells, thought to be secondary to the immunodeficiency resulting from the HTLV‐I infection. A few cases with a histological pattern resembling Hodgkin disease or angioimmunoblastic T‐cell lymphoma have been described.14,15
Figure 3 Section from a lymph node of a patient with adult T‐cell leukaemia/lymphoma, showing infiltration by lymphoid cells. Cytology is pleomorphic with the presence of cells of different sizes and with nuclear irregularities. A few immunoblast‐like cells are present. H&E stain.
Overall, the lymph node histology of ATLL is not distinctive and the histological features may be indistinguishable from those of other peripheral T‐cell lymphomas.16,17
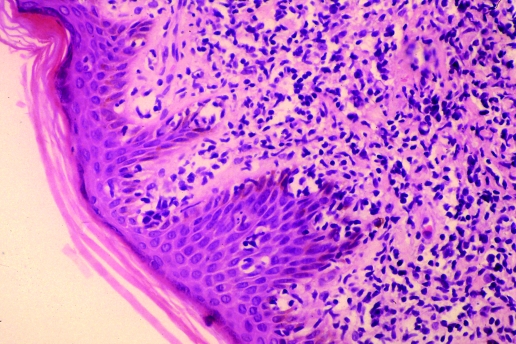
Skin histology shows lymphoid infiltrates in the dermis; in up to a half of the cases, epidermotropism is present, forming Pautrier's microabcesses with a pattern indistinguishable from that of mycosis fungoides and Sézary syndrome (SS)8 (fig 4).
Figure 4 Skin biopsy from a patient with adult T‐cell leukaemia/lymphoma, showing a dermal infiltrate with epidermotropism and Pautrier's microabcesses. H&E stain.
Immunophenotype
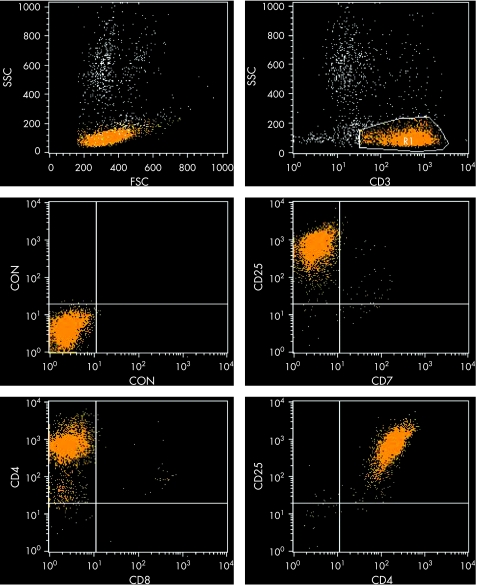
The immunophenotype of the ATLL cell is that of an activated mature T‐lymphocyte. The cells express CD2 and CD5 and often are CD7 negative; CD3 and T‐cell receptor (TCR)‐β may be down‐regulated (weak expression or even negative in the cell membrane); however, the cells express these proteins in their cytoplasm. The most common immunophenotypic profile is CD4+CD8−, but cases in which cells co‐express these two markers or express only CD8 have been described; it has been suggested that CD4+CD8+ cases have a worse clinical course.18 The most characteristic immunophenotypic feature of the neoplastic cell is the strong expression of the alpha chain of the interleukin‐2 (IL‐2) receptor recognised by the monoclonal antibody CD25 (fig 5). Such expression is distinctive but not unique to ATLL; it may be seen in other T‐cell malignancies, such as T‐prolymphocytic leukaemia (T‐PLL) and SS, although it is expressed at a lower density in these diseases. Other T‐cell activation markers such as CD38 and HLA‐DR are variably expressed and natural killer (NK) cell markers are, as a rule, negative.
Figure 5 Flow cytometry dot plot of peripheral lymphocytes of a patient with adult T‐cell leukaemia/lymphoma, showing a characteristic immunophenotypic profile: CD3+, CD4+, CD7−, CD8−, CD25+.
The immunophenotype in the tissues parallels that of the circulating neoplastic cells. In addition, large cells in some cases express CD30 but, with few exceptions, are negative with monoclonal antibodies against CD15, epithelial membrane antigen (EMA) and the anaplastic lymphoma kinase (ALK1). These features distinguish ATLL from Hodgkin's disease and anaplastic large cell lymphoma.
Cytogenetics and molecular biology
There is no distinct karyotypic or molecular abnormality in ATLL. Cytogenetic analysis often shows a complex karyotype, particularly in the leukaemic forms. Recurrent abnormalities include +3, +7, +21, monosomy X, deletion of chromosome Y and abnormalities of chromosomes 6 and 14q; the latter involve the 14q11 and 14q32 breakpoints where the TCR‐alpha and ‐delta chain genes (TCRA and TCRD) and the TCL1 gene, respectively, are located.19 Molecular analysis has shown, in cases with acute ATLL and in the lymphoma form, mutations of tumour‐suppressor genes CDKN2A (p16), CDKN2B (p15) and TP53 (p53). Investigation of the TP53 status is important as it has therapeutic implications (see later). The TCR chain genes are consistently clonally rearranged. Clonality can also be demonstrated in the blood or tissues by Southern blot or PCR with primers specific to the various fragments of HTLV‐I. These assays will show clonal integration of one or more proviruses in the tumour cells.20
Molecular analysis for HTLV‐I is not usually required for the diagnosis in patients with clinical features typical of ATLL and known to be HTLV‐I positive by serology. However, it has a role in patients who are seronegative for HTLV‐I but in whom there is a strong suspicion of ATLL, in the pure lymphomatous forms to distinguish them from other peripheral T‐cell lymphomas, and, in cases of smouldering ATLL, to distinguish such patients from a healthy carrier of the virus.
Diagnosis and differential diagnosis
The diagnosis of ATLL can be established by integrating the clinical and laboratory features. The morphology of the circulating neoplastic lymphocytes and the immunophenotype are very characteristic of the disease. Histology of the lymph node is not needed for diagnosis in patients with a leukaemic form of ATLL in whom cytology and immunophenotyping are typical of the disease and in whom serum antibodies to HTLV‐I are demonstrable. The diagnosis of the pure lymphomatous forms is more problematic as the histological features of ATLL overlap with those of other T‐cell lymphomas. In both leukaemic and lymphomatous forms, HTLV‐I serology is a mandatory investigation. The differential diagnosis includes other mature T‐cell malignancies, essentially the cerebriform variant of T‐PLL, mycosis fungoides and SS, other peripheral T‐cell lymphomas, not specified, and rarely Hodgkin disease and angioimmunoblastic T‐cell lymphoma. Patients with smouldering ATLL should be distinguished from healthy carriers of the virus.
Prognostic markers
The prognosis of ATLL is poor, with a median survival of less than one year for the acute and lymphoma forms. The projected 4‐year survival is estimated to be around 5% for these two clinical variants. Chronic and smouldering ATLL fare better, with a projected 4‐year survival of 26.9% and 62% respectively.21 Clinical form, age, performance status, elevation of LDH, high β2‐microglobulin, high serum level of CD25, high serum neuron‐specific enolase, the presence of hypercalcaemia and a high proliferative rate are the main prognostic factors.22,23 In the chronic form of ATLL, the proliferative rate of the neoplastic cells measured by Ki‐67 expression, albumin levels and LDH appear to distinguish subgroups with favourable and unfavourable prognosis.24
Treatment
Treatment of ATLL remains a challenge for the clinician. Patients usually do not respond or have only transient responses to chemotherapy regimens that are effective in aggressive lymphomas.25 The chemoresistance of this disease likely relates to multiple mechanisms including over‐expression of the multi‐drug resistance protein, TP53 mutations and dysregulation of a variety of cellular (onco)‐genes in the leukaemic cells.26 The largest experience on the results of treatment comes from Japan. A few studies suggest that intensive induction therapy with growth factors support yields a higher response rate. However, most of the patients relapse and overall survival is not improved.27 Results are limited and disappointing with purine analogues such as pentostatin (2′‐deoxycoformycin) and inhibitors of topoisomerase I and II28 and arsenic trioxide.
Monoclonal antibodies against the IL‐2 receptor (anti‐Tac), either labelled or unlabelled (daclizumab), have been used, particularly in patients with relapsed or refractory disease, but their efficacy appear to be limited, with some patients achieving partial responses and complete responses being rare.29,30 Data with a monoclonal antibody against CD52 (alemtuzumab, MabCampath), which has shown remarkable activity in T‐PLL, are scanty and limited to single case reports or reports of a few patients.31,32 However, some of the responders to this antibody were resistant to antiretroviral treatment and had TP53 abnormalities.32 The role of antibody therapy to eliminate minimal residual disease or for consolidation and maintenance of responses has not been explored.
During the last decade, a major advance on the management of ATLL came with the use of an antiretroviral—zidovudine—combined with interferon alpha. Following the two initial phase II studies that showed a high response rate in previously untreated patients,33,34 two prospective trials in France and in England confirmed the efficacy of this combination, with response rates ranging from 65% to 92% and with more than half of the patients achieving a complete response.35,36 In addition, the British study showed an improvement in overall survival compared with historical controls (median 18 months) and a significant longer survival in the responders (55% of patients alive at 4 years) versus non‐responders (median survival 6 months).35 The mechanism of action of zidovudine has been debated, as to whether it acts through inhibition of HTLV‐I replication or has a direct antiproliferative effect on the cells. A recent study has shown that in vitro long‐term treatment of HTLV‐I‐positive cell lines and fresh ATLL cells with zidovudine results in the inhibition of telomerase function, with the cells becoming senescent rather than apoptotic; such a long exposure leads to the reactivation of normal TP53 function. Furthermore, it would appear that lack of mutation of the TP53 gene predicts for response to zidovudine. Thus, in a retrospective study, patients carrying a wild‐type TP53 gene responded to this agent, while no responses were seen in patients with a mutated gene.37,38
Stem‐cell transplantation has been used in young ATLL patients in an attempt to improve and prolong response. In most of these studies, the patients received grafts from HLA identical siblings.39,40 A recent study used grafts from unrelated donors in 8 patients41; 5 of these were alive at the time of the report. Median disease free survival was 16.5 months and the overall median survival 20 months. The main causes of mortality or failure of this procedure in these patients were transplantation complications and graft‐versus‐host disease. However, allogeneic stem cell transplantation is only applicable to the small cohort of ATLL patients who are young. Allotransplantation with reduced‐conditioning intensity is an attractive alternative that could be used in older patients. A phase I clinical trial on 16 ATLL patients with a median age of 57 years (range 51–67) showed this to be a feasible treatment in this population of patients and suggested the presence of a possible graft‐versus‐leukaemia effect.42
Take‐home messages
Adult T‐cell leukaemia/lymphoma is a unique T‐cell neoplasm aetiologically linked to the human T‐cell lymphotropic virus HTLV‐1.
The disease has distinct clinical and laboratory features; it is classified into four clinical forms, the acute form being the most common.
The prognosis is poor, with refractoriness to treatment and a median survival of <1 year for the acute and lymphoma forms.
The best responses are achieved with a combination of zidovudine, interferon and chemotherapy. Consolidation with stem cell transplant should be considered in young patients.
Patients with ATLL are severely immunocompromised and should receive prophylaxis to prevent opportunistic infections due to Pneumocystis jiroveci, herpes and fungi with co‐trimoxazole, acyclovir and itraconazole or fluconazole. In addition, a screen for Strongyloides stercoralis is recommended; if the parasite is found, treatment with thiobendazole or albendazole is indicated.
In conclusion, despite the advances in the knowledge of the molecular and oncogenic mechanisms involved in the development of ATLL in HTLV‐I infected individuals, the outlook of this disease remains dismal and the choice of treatment controversial. It seems that the best results are achieved with a combination of antiretroviral drugs (zidovudine), interferon and chemotherapy. This combination should be considered the first line treatment in these patients. In young patients, responses should be consolidated with conventional or reduced conditioning intensity regimes and stem‐cell transplantation.
Footnotes
Competing interests: None.
References
- 1.Kikuchi M, Jaffe E S, Ralfkiaer E. Adult T‐cell leukaemia/lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization classification of tumours. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001200–203.
- 2.Matutes E, Catovsky D. Adult T cell leukaemia lymphoma. In: Whittaker JA, ed. Leukaemia, 3rd edn. Oxford: Blackwell Scientific Publications 1998416–433.
- 3.Shimomaya M. Diagnostic criteria and clinical subtypes of ATLL. A report from the Lymphoma Study Group (1984–87). Br J Haematol 199179428–437. [DOI] [PubMed] [Google Scholar]
- 4.Hattori T, Asou N, Suzushima H.et al Leukaemia of novel gastrointestinal T‐lymphocyte population infected with HTLV‐I. Lancet 199133776–77. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera M E, Labra S, Ford A.et al HTLV‐I induced intestinal lymphoma. Leuk Lymphoma 199935637–640. [DOI] [PubMed] [Google Scholar]
- 6.Dungerwalla M, Osuji N, Waldman A D.et al Isolated central nervous system involvement in adult T‐cell lymphoma/leukaemia. Br J Haematol 2005130511–515. [DOI] [PubMed] [Google Scholar]
- 7.Taylor G. Molecular aspects of TLV‐I infection and ATLL. J Clin Pathol 2007601392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pombo de Oliveira M S, Matutes E, Famadas L C.et al Adult T‐cell leukaemia/lymphoma in Brazil and its relation to HTLV‐I. Lancet 1990336987–990. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Sung Yul L, Shimizu T.et al Concurrence of lymphoma type adult T‐cell leukemia in three sisters. Cancer 1985561688–1690. [DOI] [PubMed] [Google Scholar]
- 10.Matutes E, Spittle M F, Smith N P.et al The first report of familial adult T‐cell leukaemia lymphoma in the United Kingdom. Br J Haematol 199589615–619. [DOI] [PubMed] [Google Scholar]
- 11.Cartier L, Castillo J L, Cabrera M E.et al HTLV‐I positive spastic paraparesis (TSP) associated with a lymphoid disorder in three Chilean patients. Leuk Lymphoma 199517459–464. [DOI] [PubMed] [Google Scholar]
- 12.Aouba A, Lambotte O, Vasiliu V.et al Hemophagocytic syndrome as a presenting sign of transformation of smouldering to acute adult T‐cell leukemia/lymphoma: efficacy of anti‐retroviral and interferon therapy. Am J Hematol 200476187–189. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Matutes E, Catovsky D.et al Strongyloides stercoralis as candidate cofactor for HTLV‐I induced malignancies. Lancet 1987ii94–95. [DOI] [PubMed] [Google Scholar]
- 14.Duggan D, Ehrlich G, Davey F.et al HTLV‐I induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV‐I sequences in tumor DNA. Blood 1988711027–1032. [PubMed] [Google Scholar]
- 15.Oshima K, Kikuchi M, Yoshida T.et al Lymph nodes in incipient adult T‐cell leukemia‐lymphoma with Hodgkin's disease like histologic features. Cancer 1990671622–1628. [DOI] [PubMed] [Google Scholar]
- 16.Lennert K, Kikuchi M, Sato E.et al HTLV‐positive and ‐negative T‐cell lymphomas. Morphological and immunohistochemical differences between European and HTLV‐positive Japanese T‐cell lymphomas. Int J Cancer 19853565–72. [DOI] [PubMed] [Google Scholar]
- 17.Ohshima K, Suzumiya J, Sato K.et al Nodal T‐cell lymphoma in an HTLV‐1 endemic area: proviral HTLV‐1 DNA, histological classification and clinical evaluation. Br J Haematol 1998101703–711. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Unoki T, Sagawa K.et al Clinical features of OKT4+/OKT8+ adult T‐cell leukemia. Leuk Res 198591353–1359. [DOI] [PubMed] [Google Scholar]
- 19.Fifth International Workshop on Chromosomes in Leukemia–lymphoma Correlation of chromosome abnormalities with histologic and immunologic characteristics in non‐Hodgkin's lymphoma and adult T‐cell leukemia lymphoma. Blood 1988701554–1564. [PubMed] [Google Scholar]
- 20.Yamaguchi K. Human T‐lymphotropic virus type I in Japan. Lancet 1994343213–216. [DOI] [PubMed] [Google Scholar]
- 21.Shimoyama M. Treatment of patients with adult T‐cell leukemia‐lymphoma: an overview. In: Takatsuki K, Hinuma Y, Yoshida M, eds. Advances in adult T‐cell leukemia and HTLV‐I research. Tokyo: Japan Scientific Societies Press, 19923943–56. [Google Scholar]
- 22.Sadamori N, Mine M, Hakariya S.et al Clinical significance of B2‐microglobulin in serum of adult T cell leukemia. Leukemia 19959594–597. [PubMed] [Google Scholar]
- 23.Nishimura S, Asou N, Suzushima H.et al P53 gene mutation and loss of heterozygosity are associated with increased risk of disease progression in adult T cell leukemia. Leukemia 19959598–604. [PubMed] [Google Scholar]
- 24.Shirono K, Hattori T, Takatsuki K. A new classification of clinical stages of adult T‐cell leukemia based on prognosis of the disease. Leukemia 199481834–1837. [PubMed] [Google Scholar]
- 25.Bazarbachi A, Ghez D, Lepelletier Y.et al New therapeutic approaches for adult T‐cell leukaemia. Lancet Oncol 20045664–672. [DOI] [PubMed] [Google Scholar]
- 26.Taylor G P, Matsuoka M. Natural history of adult T‐cell leukemia/lymphoma and approaches to therapy. Oncogene 2005246047–6057. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Tomonaga M, Fukuda H.et al A new G‐CSF‐supported combination chemotherapy, LSG15, for adult T‐cell leukaemia‐lymphoma. Br J Haematol 2001113375–382. [DOI] [PubMed] [Google Scholar]
- 28.Mercieca J, Matutes E, Dearden C.et al The role of pentostatin in the treatment of T‐cell malignancies: analysis of response rate in 145 patients according to disease subtype. J Clin Oncol 1994122588–2593. [DOI] [PubMed] [Google Scholar]
- 29.Waldmann T A, White J D, Goldman C K.et al The interleukin‐2 receptor: a target for monoclonal antibody treatment of human T‐cell lymphotropic virus‐I induced adult T‐cell leukemia. Blood 1993821701–1712. [PubMed] [Google Scholar]
- 30.Waldmann T A, White J D, Carrasquillo J A.et al Radio‐immunotherapy of interleukin‐2R alpha expressing adult T‐cell leukemia with yttrium‐90‐labeled anti‐Tac. Blood 1995864063–4075. [PubMed] [Google Scholar]
- 31.Ravandi F, Faderl S. Complete response in a patient with adult T‐cell leukemia (ATL) treated with combination of alemtuzumab and pentostatin. Leuk Res 200630103–105. [DOI] [PubMed] [Google Scholar]
- 32.Mone A, Puhalla S, Whitman S.et al Durable hematologic complete response and suppression of HTLV‐1 viral load following alemtuzumab in zidovudine/IFN‐a‐refractory adult T‐cell leukemia. Blood 20051063380–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill P S, Harrington W, Jr, Kaplan M H.et al Treatment of adult T‐cell leukemia‐lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 19953321744–1748. [DOI] [PubMed] [Google Scholar]
- 34.Hermine O, Bouscary D, Guessain A.et al Treatment of HTLV‐1 associated adult T‐cell leukemia‐lymphoma with a combination of zidovudine and alpha‐interferon. N Engl J Med 19953321749–1751. [DOI] [PubMed] [Google Scholar]
- 35.Matutes E, Taylor G P, Cavenagh J.et al Interferon alpha and zidovudine therapy in adult T‐cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol 2001113779–784. [DOI] [PubMed] [Google Scholar]
- 36.Hermine O, Allard I, Levy V.et al A prospective phase II clinical trial with the use of zidovudine and interferon‐alpha in the acute and lymphoma forms of adult T‐cell leukemia/lymphoma. Haematology J 20023276–282. [DOI] [PubMed] [Google Scholar]
- 37.Mahieux R. HTLV‐I and AZT: die another day. Blood 2006108785–786. [Google Scholar]
- 38.Datta A, Bellon M, Sinha‐Datta U.et al Persistent inhibition of telomerase reprograms adult T‐cell leukemia to p53‐dependent senescence. Blood 20061081021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utsunomiya A, Miyakazi Y, Takatsuka Y.et al Improved outcome of adult T‐cell leukemia/lymphoma by allogeneic stem cell transplantation. Bone Marrow Transplant 20012715–20. [DOI] [PubMed] [Google Scholar]
- 40.Kami M, Hamaki T, Miyakoshi S.et al Allogeneic haematopoietic stem cell transplantation for the treatment of adult T‐cell leukaemia/lymphoma. Br J Haematol 2003120304–309. [DOI] [PubMed] [Google Scholar]
- 41.Nakase K, Hara M, Kozuka T.et al Bone marrow transplantation from unrelated donors for patients with adult T‐cell leukaemia/lymphoma. Bone Marrow Transplant 20063741–44. [DOI] [PubMed] [Google Scholar]
- 42.Okamura J, Utsunomiya A, Tanosaki R.et al Allogeneic stem‐cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T‐cell leukemia/lymphoma. Blood 20051054143–4145. [DOI] [PubMed] [Google Scholar]