Abstract
This study aims to review histological and immunohistochemical features that are useful in the diagnosis of metastases to the breast. Histological features were compared between non‐haematological metastases to the breast and 100 consecutive core biopsy specimens of primary invasive carcinomas of the breast. 18 non‐haematological metastases to the breast were diagnosed over a 10‐year period (0.3% of malignant mammary tumours). Elastosis and carcinoma in situ were seen only in primary mammary cancers. Two‐thirds of tumours had features raising the possibility of metastasis, such as clear cell carcinoma suggestive of renal origin and small cell carcinoma suggestive of pulmonary origin. The features observed in haematological metastases are also described. Immunohistochemical panels to distinguish mammary carcinoma (oestrogen receptor, gross cystic fluid protein‐15) from common metastases to the breast, including carcinoma of the lung (thyroid transcription factor‐1), malignant melanoma (S100, HMB45, melan‐A) and ovarian serous papillary carcinoma (Wilms' tumour 1), are discussed. The pathologist has a key role in considering the diagnosis of metastasis to the breast if the histological features are unusual for a primary mammary tumour. The clinical history is vital in some cases. Immunohistochemistry plays a useful supplementary role.
The diagnosis of metastases to the breast from extramammary malignancies, and distinction from primary mammary malignancy, is important for patient management. The prognosis is generally poor as most patients have widely disseminated disease.1,2,3 Most patients die within a year of diagnosis,2,4,5,6,7 although longer survival is well recognised if there is effective systemic treatment.2,6 In many patients, systemic treatment or palliative care is more appropriate than extensive surgery. Accurate diagnosis can therefore prevent unnecessary surgery.
The aim of this paper is to describe histological and immunohistochemical features that are useful in the diagnosis of the common tumour types that metastasise to the breast. There is increasing use of needle‐core biopsy rather than fine‐needle aspiration cytology in non‐operative diagnosis of breast disease. The emphasis in this article is on the diagnosis in needle‐core biopsy: if a diagnosis can be made at this stage then appropriate management can be planned.
The most common metastatic tumours in the breast are from mammary primaries,8,9 but these are excluded in most series and will not be discussed here. A wide range of extramammary tumours have been described as metastasising to the breast, the largest group being haematological malignancies. Other common types are carcinoma of the lung, malignant melanoma, serous papillary carcinoma of the ovary, carcinoma of the prostate, kidney and stomach, and carcinoid tumours.10 Malignant tumours of the breast are rare in people aged <20 years. In this small group, metastases to the breast outnumber primary tumours, and the most common tumours metastasising to the breast are rhabdomyosarcomas and lymphomas.11,12 Metastases to the breast are much more common in women.9
The frequency of metastastic tumour in the breast from extramammary malignancy compared with primary mammary carcinoma, based on histological diagnosis in clinical studies, varies between 0.2% and 1.3%.4,5,7 Higher frequencies of 2–7% are seen in postmortem studies.8,13 In approximately 30% of patients, the metastasis to the breast is the first sign of malignancy.1,4,5,6,7,9 In those with a history of malignancy, the time from initial diagnosis to metastasis to the breast varies between 1 month and 15 years, with averages between 1 and 5 years.1,2,5,14,15,16 A long interval is well recognised for some tumour types such as malignant melanoma and ovarian carcinoma (table 1).5
Table 1 Clinical and histological features of 18 patients with metastases to the breast seen in Nottingham City Hospital, Nottingham, UK, 1996–2005.
| Primary site | Histological pattern | Age (years) | Sex | Time from diagnosis to breast metastasis (months) | Calcification | Distinctive pathology |
|---|---|---|---|---|---|---|
| Lung | Small cell carcinoma | 49 | F | 13 | No | Yes, small cell |
| Lung | Squamous carcinoma | 83 | F | 10 | No | No |
| Lung | Large cell carcinoma | 58 | M | 3 | No | No |
| Lung | Small cell carcinoma | 49 | F | 9 | No | Yes, small cell |
| Lung | Adenocarcinoma | 64 | F | 3 | No | Yes, unusual pattern |
| Ovary | Serous papillary carcinoma | 58 | F | 9 | Yes | No |
| Ovary | Serous papillary carcinoma | 71 | F | 93 | Yes | Yes, papillary |
| Ovary | Serous papillary carcinoma | 70 | F | 94 | Yes | Yes, papillary |
| Ovary | Serous papillary carcinoma | 72 | F | New diagnosis | No | Yes, papillary |
| Skin | Melanoma | 29 | F | 16 | No | No |
| Skin | Melanoma | 67 | F | 118 | No | Yes, spindle cells, pigment |
| * | Melanoma | 73 | M | * | No | Yes, spindle cells |
| Small bowel | Melanoma | 42 | F | 9 | No | Yes, intranuclear inclusions |
| Oesophagogastric | Diffuse carcinoma | 60 | F | 12 | No | No, like lobular |
| Kidney | Clear cell carcinoma | 58 | F | 2 | No | Yes, clear cell |
| Prostate | Adenocarcinoma | 75 | M | 68 | No | No |
| Thyroid | Hurtle cell carcinoma | 53 | F | New diagnosis | No | Yes, abundant granular cytoplasm |
| Ovary | Leiomyosarcoma | 61 | F | 13 | No | Yes, spindle cells |
F, female; M, male.
*Information not obtainable.
Clinical features
Patients typically present with a rapidly growing painless firm palpable breast mass.1,2,7,14,15,16,17 Some reports emphasise that the masses are often superficial,4,18 but usually they are not tethered to the skin.14,15,17 Diffuse skin involvement is rare.
The most common mammographic appearance is of a rounded mass with well‐defined or slightly irregular margins.7,15,18,19 Multiple or bilateral tumours are seen in a minority. Calcification is rare, apart from metastases from ovarian serous papillary carcinomas.1,6,20 Spiculation is uncommon in contrast with primary mammary carcinomas.15,20 Ultrasound scan typically shows a hypoechoic mass, which is sometimes heterogeneous or poorly defined.18 Axillary lymphadenopathy is sometimes apparent.
Useful histological features
The histological features in 18 non‐haematological metastases to the breast seen over a 10‐year period (1996–2005) were compared with a nearly consecutive series of 100 core biopsy specimens in 2006, showing invasive carcinoma with later surgical excision (table 2), and larger series of core biopsy21 and excision specimens.22
Table 2 Comparison of histological features between non‐haematological metastases to the breast and primary mammary carcinomas.
| Histological feature | Primary mammary carcinoma | Metastases to the breast | |||
|---|---|---|---|---|---|
| Core biopsy specimens21 | Core biopsy specimens, NCH 2006 | Excision, NCH 2006 | Excision22 | Core biopsy specimens*, NCH 1996–2005 | |
| Number of specimens | 500 | 100 | 100 | 745 | 18 |
| Carcinoma in situ (%) | NS | 43 | 80 | 87 | 0 |
| DCIS (%) | 32 | 40 | 78 | NS | 0 |
| Lobular neoplasia (%) | NS | 3 | 10 | NS | 0 |
| Elastosis (%) | NS | 51 | NS | 40 | 0 |
| Calcification (%) | NS | 19 | NS | NS | 17† |
| Vascular invasion (%) | 3 | NS | NS | 26 | 0 |
DCIS, ductal carcinoma in situ; NCH, Nottingham City Hospital; NS, not studied.
*Includes one excision specimen.
†All ovarian serous papillary carcinomas.
Often metastases to the breast show histological features, such as clear cell carcinoma suggestive of renal origin, which are not typical of primary carcinoma of the breast (table 1). The clues can sometimes be subtle such as pigment and intranuclear inclusions in malignant melanoma. In our experience, about a third of lesions do not show specific histological features (table 1). For example, large cell carcinoma of the lung may resemble grade 3 invasive ductal carcinoma of the breast. A history is often essential to make a correct diagnosis in such patients.
Elastosis is common in primary mammary carcinomas, but rare in extramammary tumours (table 2). The presence of carcinoma in situ strongly supports the diagnosis of primary carcinoma (table 2), but can be very rarely seen in association with metastasis from an extramammary primary carcinoma.23 Calcification is common in mammary carcinomas, but is rarely seen in metastases to the breast with the exception of serous papillary carcinoma of the ovary or peritoneum (table 2).
Four growth patterns of metastases to the breast are described. The most common one is a circumscribed nodule with surrounding normal breast tissue.5,7 Infiltration around ducts and lobules is particularly associated with lymphomas, leukaemias and malignant melanoma.1,2,13,16 This pattern has been suggested to be a clue to the diagnosis of metastasis to the breast, but it can be seen in primary breast tumours. It was only apparent in haematological malignancies in the tumours from Nottingham City Hospital. Lymphangitis and diffuse infiltration are less‐common patterns. These growth patterns are less easy to appreciate in a core biopsy than in surgical specimens.
Immunohistochemistry
The most useful data in making the diagnosis of metastasis to the breast are the clinical history and morphological assessment of haematoxylin and eosin (H&E)‐stained sections, particularly if sections of the primary tumour are available for comparison. When considering a possible diagnosis of metastasis to the breast from a known malignancy elsewhere, it is important to ask oneself whether the extramammary tumour may in fact be a metastasis from the breast tumour. If there is no history, immunohistochemical analysis may be helpful in supporting origin from an extramammary site. An immunohistochemical comparison with a known extramammary primary tumour using a panel of antibodies may be useful in small biopsies with limited tissue for assessment or tumours without distinctive histology, which could be either a primary mammary tumour or metastasis from an extramammary malignancy.
When performing immunohistochemical analysis it is important to remember that no marker is 100% specific or sensitive. Thus, one should use panels of antibodies and not rely too much on any individual result. There is a danger of false‐negative results in small biopsies, particularly if the antigen is only focally present. Also, metastases sometimes show a different immunophenotype from the primary tumour, but usually for just one or two markers. Important contributory factors to the percentage of positive results for each antibody discussed below are technical details (fixation, processing, pretreatment and immunohistochemical method), criteria for a positive result and selection of tumours. The percentage of tumours described as expressing different markers in the sections below must therefore be regarded as approximate. The choice of antibodies used should be based on the history and morphology of the tumour.
Immunophenotype of breast cancer
The combination of cytokeratin 7 and cytokeratin 20 is useful in categorising carcinomas (table 3).24,25 Breast cancer, including the common special types, is typically cytokeratin 7+ and cytokeratin 20−.26 Almost all breast cancers stain with the cytokeratin antibody CAM5.2 and are positive for epithelial membrane antigen.27,28 S100 is expressed in 50%29,30 and carcinoembryonic antigen in 30% of mammary carcinomas.31 Oestrogen receptor is expressed in 80% and progesterone receptor in 60% of mammary carcinomas,32,33,34 with most tumours being either clearly positive or completely negative.34 Convincing expression of oestrogen receptor is largely restricted to carcinomas of the breast, endometrium and ovary.35 Occasionally, tumours from other sites express oestrogen receptor, but usually it is weak and focal.35,36,37 Gross cystic disease fluid protein‐15 (GCDFP‐15) is often expressed by carcinomas of the breast (70%), salivary glands and skin appendages and occasionally by other carcinomas.36,38,39
Table 3 Predominant patterns of expression of cytokeratin 7 and cytokeratin 20 in carcinomas arising in different organs.
| Immunophenotype | Organ of origin/histological type |
|---|---|
| CK7+/CK20− | Breast carcinoma |
| Non‐mucinous ovarian carcinoma | |
| Pulmonary adenocarcinoma | |
| Endometrium | |
| Pleural mesothelioma | |
| Thyroid carcinoma | |
| Oesophageal adenocarcinoma | |
| Salivary gland | |
| CK7−/CK20+ | Colorectal |
| CK7±/CK20+ | Gastric |
| CK7+/CK20± | Pancreas/biliary |
| CK7+/CK20+ | Mucinous ovary |
| Transitional cell | |
| CK7−/CK20− | Prostate |
| Renal clear cell carcinoma | |
| Hepatocellular | |
| Pulmonary squamous carcinoma |
Ovarian carcinoma
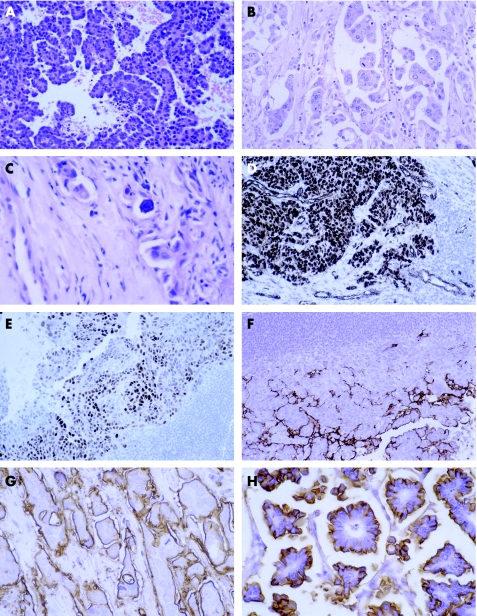
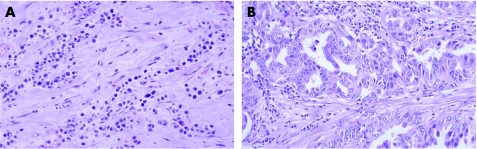
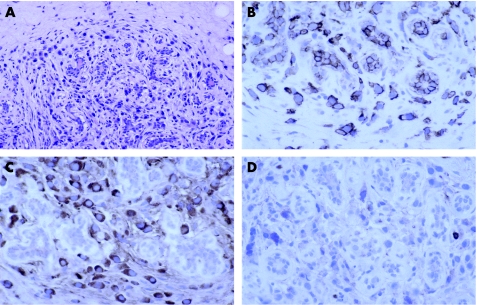
Serous papillary carcinoma is the most common type of ovarian tumour to the metastasise to the breast and can also involve axillary lymph nodes.40 Histologically, it is not possible to distinguish it from serous papillary carcinoma of the peritoneum, which can also spread to the breast. Usually, the papillary architecture is apparent, but sometimes there may be just a solid growth pattern making diagnosis more difficult. Occasionally, the time from initial diagnosis of ovarian primary to mammary metastasis is several years. The histological clue to the diagnosis is that the papillary architecture is not a typical pattern for most histological types of invasive carcinoma of the breast (fig 1A,B). Serous papillary carcinoma can resemble invasive micropapillary carcinoma of the breast and calcification can be seen in both.
Figure 1 Metastasis from serous papillary carcinoma of the ovary: (A) typical papillary architecture; (B) less typical papillary architecture and (C) calcification. Immunohistochemical analysis shows expression of (D) Wilms' tumour 1 in tumour nuclei and vessels, (E) oestrogen receptor and (F) Ca125. Epithelial membrane antigen expression in (G) metastasis from serous papillary carcinoma of the ovary compared with (H) invasive micropapillary carcinoma of the breast.
Both mammary and non‐mucinous ovarian carcinomas are typically cytokeratin 7+, cytokeratin 20− and often positive for oestrogen receptor. The pattern of epithelial membrane antigen expression is useful: invasive micropapillary carcinoma has expression on the outside of the papillary clusters, but not around the central spaces, whereas serous papillary carcinoma has expression on both surfaces (fig 1G,H).
Nuclear expression of Wilms' tumour 1 is present in about 70% of ovarian carcinomas and in 95% of serous papillary carcinomas (fig 1D), but is present in <10% of breast cancers (although there do not seem to be any data for invasive micropapillary carcinoma).41,42,43,44 It is also present in other tumours such as mesothelioma.45
GCDFP‐15 is present in about 70% of breast cancers, including invasive micropapillary carcinomas,46 and rarely seen in ovarian carcinoma.35,36,38,39,44 Thus, expression of this marker favours breast cancer and the absence of staining is not helpful.
Staining for Ca125 is seen in about 60% of ovarian carcinomas and in 90% of serous papillary carcinomas (fig 1F). It is also commonly seen in endometrial, endocervical, biliary and pancreatic carcinomas, but infrequently in breast cancer (10–20%).35,39,44,47
Mesothelin is often expressed in carcinomas of the ovary (over 90% of serous papillary carcinomas), prostate and mesotheliomas and weakly expressed in 3–14% of breast cancers.35,48,49 Intermediate levels of staining are seen in adenocarcinomas of the lung, stomach and colorectum.
Malignant melanoma
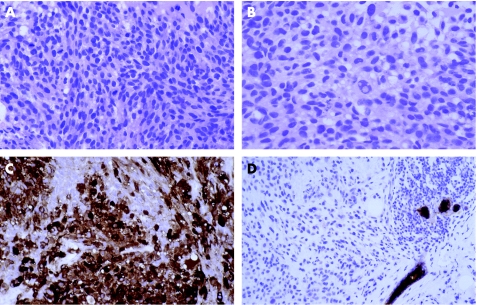
The histological appearance of malignant melanoma can be varied, including epithelioid, spindle and plasmacytoid cells, and may overlap with mammary carcinoma (table 1).50 Useful clues to the diagnosis are cytoplasmic pigment, intranuclear inclusions and spindle cells (fig 2A,B).
Figure 2 Metastases from malignant melanoma with (A) spindle cells and (B) intranuclear inclusions. Immunohistochemical analysis shows expression of (C) S100 and (D) absence of cytokeratin expression (note the positive internal control).
S100 is the most sensitive immunohistochemical marker of melanoma (expressed in about 95%, fig 2C). However, it is not specific, being present in many other tumours, including about 50% of breast cancers,29,30 and must therefore be used in combination with other markers. A panel of cytokeratins is useful to exclude carcinoma (fig 2D). HMB45, melan‐A, microphthalmia transcription factor and tyrosinase are all less sensitive, being present in about 70% of melanomas, and more specific than S100.51,52,53 Melanoma can show aberrant expression of cytokeratins, particularly with CAM5.2, epithelial membrane antigen, CD38 and CD68.50
Pulmonary carcinoma
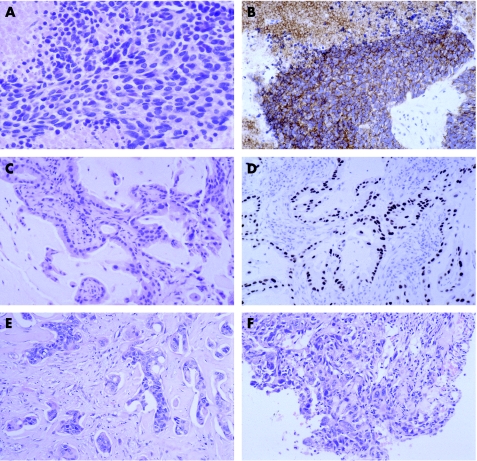
The major clue to the diagnosis of oat cell carcinoma is the appearance on H&E‐stained sections. It is typically composed of sheets of cells with speckled chromatin without prominent nucleoli, scant cytoplasm, necrosis, frequent mitoses and crush artefact (fig 3A). Immunohistochemistry is useful to confirm the diagnosis. Membranous staining for CD56 is present in 95% (fig 3B) and other neuroendocrine markers, such as synaptophysin and chromogranin A, are less frequently seen.54 There is typically dot positivity with CAM5.2. Thyroid transcription factor‐1 (TTF‐1) is present in about 80% of both pulmonary and non‐pulmonary small cell carcinomas (and can be seen in primary mammary small cell carcinoma).55,56 The possibility of metastasis, particularly from the lung, should be considered if small cell carcinoma is diagnosed in the breast, as primary mammary small cell carcinoma is very rare.57 The presence of ductal carcinoma in situ or in oestrogen receptor favours the diagnosis of primary mammary small cell carcinoma.56,57
Figure 3 Metastases from pulmonary carcinomas. Small cell carcinoma (A) H&E and (B) CD56. Adenocarcinoma (C) H&E and (D) thyroid transcription factor 1. (E) Squamous carcinoma. (F) Large cell carcinoma.
Pulmonary adenocarcinomas may have morphological clues such as an acinar growth pattern or mucin‐secreting columnar cells (fig 3C). TTF‐1 is expressed by about 75% of pulmonary adenocarcinomas (fig 3D) and, apart from carcinomas of the lung and thyroid, it is rarely seen in other carcinomas.35,45,55,58 TTF‐1‐positive conventional mammary carcinoma can rarely be seen (Colin Purdie, personal communication). Expression of oestrogen receptor and GCDFP‐15 favour a primary breast carcinoma although convincing expression is seen occasionally in pulmonary adenocarcinomas.35,36,37
Primary keratinising squamous carcinoma of the breast is very rare, so metastasis, particularly from the lung, needs to be considered with this histological appearance. The recently recognised basal carcinoma of the breast can show squamoid differentiation without keratinisation59,60—such tumours are typically grade 3, express basal keratins such as cytokeratin 14 and are often negative for oestrogen receptor, progesterone receptor and HER‐2.61 TTF‐1 is rarely, if ever, present in pulmonary squamous carcinoma.58 Owing to this overlap in morphology and immunophenotype, the clinical history may be essential for making the correct diagnosis of metastasis from extramammary non‐keratinising squamous carcinoma (fig 3E).
Metastasis from large cell carcinoma of the lung and poorly differentiated breast cancer are difficult to distinguish on H&E‐ stained sections (fig 3F). Some large cell carcinomas of the lung express TTF‐1.62 Expression of oestrogen receptor and GCDFP‐15 favours breast cancer. Clinical history and comparison with previous histology may be needed to make an accurate diagnosis.
Prostate
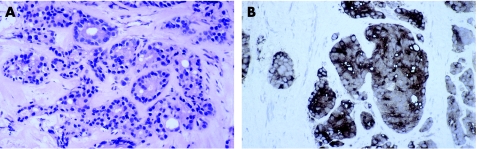
The morphology of prostatic carcinoma overlaps with mammary carcinoma (fig 4, table 1). Prostatic carcinoma may have columnar cells and even if the nuclei are relatively bland they typically contain a nucleolus. Prostate‐specific antigen and prostatic acid phosphatase are excellent markers of prostatic carcinoma as both are expressed in nearly 100% of tumours (fig 4).35,63,64,65 Apart from tumours of the salivary gland,66,67 few other tumours express these markers. Recent reports suggest that male breast cancers can express prostate‐specific antigen (15%) but not prostatic acid phosphatase.68,69,70 Oestrogen receptor, GCDFP‐15 and cytokeratin 7 are uncommon in prostatic carcinoma,35,65 so expression of these markers favours breast cancer.
Figure 4 Metastasis from prostatic carcinoma (A) H&E (B) prostate‐specific antigen.
Stomach
The intestinal pattern of gastric carcinoma may resemble invasive ductal carcinoma of the breast, and diffuse gastric carcinoma may resemble invasive lobular carcinoma of the breast (fig 5). Columnar mucin‐secreting cells favour gastrointestinal origin. Some earlier reports describe oestrogen‐receptor‐positive gastric carcinoma, but recent studies suggest that this is rare.35,71,72,73 Oestrogen receptor is expressed by 95% of invasive lobular carcinomas,32,34 so this marker is particularly useful in the distinction from diffuse gastric carcinoma. Several recent studies did not find GCDFP‐15‐positive stomach cancer.35,71,73 CDX2 is present in between 20% and 70% of gastric carcinomas but not in breast cancer.35,73,74,75 Cytokeratin 20 is more often present in gastric carcinoma (50%) than in mammary carcinoma.
Figure 5 (A) Metastasis from gastrooesophageal carcinoma of diffuse pattern. (B) The primary carcinoma also had areas of intestinal carcinoma. The diagnosis was reinforced by the absence of expression of oestrogen receptor.
Renal cell carcinoma
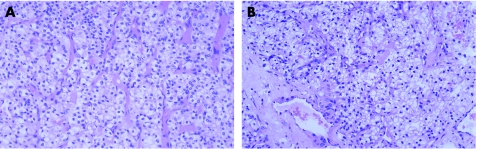
Conventional renal cell carcinoma is the most common renal malignancy and the most likely to metastasise to a wide range of sites76 including the breast. The abundant clear or granular cytoplasm with prominent fine vessels are useful clues to this diagnosis (fig 6). Clear cell change can be seen in mammary carcinoma, but is often patchy, and in carcinomas from other sites.
Figure 6 (A) Primary conventional renal cell carcinoma and (B) mammary metastasis. The similarity in morphology means that no immunohistochemical analysis is necessary.
Conventional renal cell carcinoma is usually positive for the renal cell carcinoma marker (90%), whereas only about 15% of breast cancers are positive,77 and stromal cells could be positive.78 CD10 is present in a high proportion of conventional and papillary renal cell carcinomas (90%), commonly seen in other genitourinary and gastrointestinal tumours, but is uncommon in breast cancer (5%).79 Oestrogen receptor, GCDFP‐15 and cytokeratin 7 are rarely expressed in conventional renal cell carcinoma,35,36,80 although cytokeratin 7 is more common in other histological types.81
Carcinoid tumours
Carcinoid tumours of the small bowel and appendix metastasise to the breast surprisingly commonly.10,15,17 Primary endocrine carcinomas of the breast and carcinoid tumours of the lung and gastrointestinal tract can be morphologically similar. Ductal carcinoma in situ is a useful discriminant in breast biopsy specimens.
Immunohistochemical analysis can provide some clues to the primary site of carcinoid tumours. Expression of CDX2 and CK20 favours gastrointestinal origin and TTF‐1 favours pulmonary origin.55,74,82 There seem to be no data on these three markers in breast neuroendocrine tumours. Oestrogen and progesterone receptor and GCDFP‐15 are often expressed by mammary neuroendocrine carcinomas.83 Progesterone receptor is expressed in some pancreatic endocrine tumours, but not in gastrointestinal or pulmonary carcinoid tumour84; oestrogen receptor is not expressed in any of these extramammary tumours.
Sarcomas
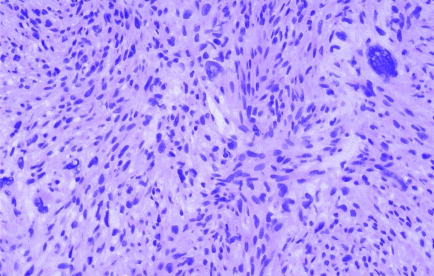
Both primary and metastatic sarcomas in the breast are rare. Sarcoma is more commonly seen as a component of metaplastic carcinoma or phyllodes tumour. The limited tissue in a core biopsy specimen makes accurate diagnosis difficult unless there is a history (fig 7). Thorough sampling, looking for areas of conventional carcinoma or small cohesive foci, and cytokeratin immunohistochemistry using a panel of antibodies are useful in diagnosing metaplastic carcinoma.85 A search for leaf‐like areas of benign epithelium and CD34 immunohistochemical analysis are helpful in diagnosing phyllodes tumour.85
Figure 7 Metastasis from leiomyosarcoma. The diagnosis was made by comparison with the ovarian primary.
Take‐home messages
Metastases to the breast need to be considered if the histological appearance is unusual for a primary mammary tumour. Two‐thirds of metastases to the breast have histological features, raising the possibility of this diagnosis.
In some cases the histological appearance is similar to a primary mammary tumour and the clinical history is essential to making the diagnosis.
Elastosis and carcinoma in situ favours primary mammary carcinoma.
Immunohistochemistry using a panel of antibodies often plays a useful supplementary role to H&E‐stained sections.
No antibody is 100% sensitive or specific for any tumour type.
Lymphomas
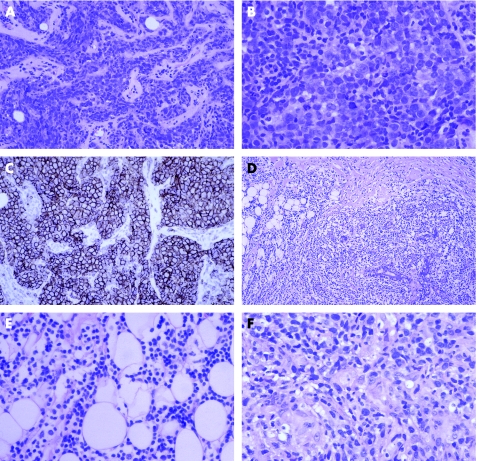
The distinction between primary and secondary lymphoma of the breast is based on clinical criteria.86,87 A wide range of mammary lymphomas have been described, but the most common type, primary or secondary, is diffuse large B cell lymphoma.86,87 This is usually readily recognised as malignant on histological examination. The major pitfall is not to consider the possibility of lymphoma and misdiagnose the tumour as carcinoma (fig 8A). The clue is the cytology of the cells, which are most commonly centroblastic and less often immunoblastic. Immunohistochemical analysis for lymphoid markers establishes the diagnosis (fig 8C).
Figure 8 Diffuse large B cell lymphoma of the breast: (A) the cohesive appearance mimics mammary carcinoma; (B) at higher power, the centroblastic morphology is apparent; and (C) CD20 confirms B cell type. (D) Marginal zone lymphoma with dense sheets of lymphocytes infiltrating around lobule and (E) at higher power the monotonous nature of the infiltrate is apparent. (F) Metastasis from mycosis fungoides showing heterogeneous infiltrate, including a small granuloma.
Other common types of secondary mammary lymphoma are follicular, marginal zone and small lymphocytic lymphoma/chronic lymphocytic leukaemia.88 In low‐grade lymphomas, the differential diagnosis is with inflammatory disorders. A diagnostic clue is the dense monotonous nature of the infiltrate (fig 8D,E). For follicular lymphoma, the important differential diagnosis is reactive germinal centres. Lymphoepithelial lesions are not restricted to marginal zone lymphoma. Immunohistochemistry and PCR for immunoglobulin heavy‐chain clones or translocations are often helpful. T cell lymphomas are uncommon89; clear cytoplasm is a useful pointer (fig 8F). If the diagnosis of lymphoma is not straightforward, a specialist opinion is recommended.
Leukaemia
Leukaemia occasionally involves the breast. The morphology of the blasts or more differentiated cells may give a clue to the diagnosis, but a high index of suspicion may be needed to make the correct diagnosis if there is no clinical history.87
Myeloma
Myeloma rarely involves the breast.87 The plasmacytic morphology and pattern of infiltration around lobules (fig 9) suggest the diagnosis. Showing light‐chain restriction is important in establishing the diagnosis (fig 9). CD38 and CD138 are useful markers of plasma cell differentiation, but neither is specific.90,91,92
Figure 9 (A) Multiple myeloma showing lobulocentric infiltrate. (B) The tumour cells are CD138+. There is λ light chain restriction (C) λ, (D) κ. There was simultaneous involvement of the bone marrow.
Conclusion
Although metastases to the breast are uncommon, accurate diagnosis is important to ensure appropriate management. The diagnosis may be straightforward if there is a clinical history of extramammary malignancy, particularly if sections are available for comparison. The pathologist has a key role in considering the possibility of metastasis if the morphology of the tumour is not typical of a primary mammary tumour. As Jane Austen said “A lucky guess is never merely luck. There is always some talent in it”.93
Abbreviations
H&E - haematoxylin and eosin
GCDFP‐15 - gross cystic disease fluid protein‐15
TTF‐1 - thyroid transcription factor‐1
Footnotes
Competing interests: None declared.
References
- 1.Toombs B D, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. Am J Roentgenol 1977129673–676. [DOI] [PubMed] [Google Scholar]
- 2.Chaignaud B, Hall T J, Powers C.et al Diagnosis and natural history of extramammary tumors metastatic to the breast. J Am Coll Surg 199417949–53. [PubMed] [Google Scholar]
- 3.Vizcaino I, Torregrosa A, Higueras V.et al Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol 2001111659–1665. [DOI] [PubMed] [Google Scholar]
- 4.Hajdu S I, Urban J A. Cancers metastatic to the breast. Cancer 1972291691–1696. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh I H, Hooper A A, Millis R R.et al Metastatic carcinoma within the breast. Clin Oncol 19762393–401. [PubMed] [Google Scholar]
- 6.McCrea E S, Johnston C, Haney P J. Metastases to the breast. Am J Roentgenol 1983141685–690. [DOI] [PubMed] [Google Scholar]
- 7.Alvarado Cabrero I, Carrera Alvarez M, Perez Montiel D.et al Metastases to the breast. Eur J Surg Oncol 200329854–855. [DOI] [PubMed] [Google Scholar]
- 8.Abrams H L, Spiro R, Goldstein N. Metastases in carcinoma. Cancer 1950374–84. [DOI] [PubMed] [Google Scholar]
- 9.Georgiannos S N, Aleong J C, Goode A W.et al Secondary neoplasms of the breast: a survey of the 20th century. Cancer 2001922259–2266. [DOI] [PubMed] [Google Scholar]
- 10.Alva S, Shetty‐Alva N. An update of tumor metastasis to the breast data.[letter]. Arch Surg 1999134450. [DOI] [PubMed] [Google Scholar]
- 11.Rogers D A, Lobe T E, Rao B N.et al Breast malignancy in children. J Pediatric Surg 19942948–51. [DOI] [PubMed] [Google Scholar]
- 12.Pettinato G, Manivel J C, Kelly D R.et al Lesions of the breast in children exclusive of typical fibroadenoma and gynecomastia. A clinicopathologic study of 113 cases. Pathol Annu 198924295–328. [PubMed] [Google Scholar]
- 13.Sandison A T. Metastatic tumours in the breast. Br J Surg 19594754–58. [DOI] [PubMed] [Google Scholar]
- 14.Amichetti M, Perani B, Boi S. Metastases to the breast from extramammary malignancies. Oncology 199047257–260. [DOI] [PubMed] [Google Scholar]
- 15.Vergier B, Trojani M, de Mascarel I.et al Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol 199148112–116. [DOI] [PubMed] [Google Scholar]
- 16.Silverman E M, Oberman H A. Metastatic neoplasms in the breast. Surg Gynecol Obstet 197413826–28. [PubMed] [Google Scholar]
- 17.Nielsen M, Andersen J A, Henriksen F W.et al Metastases to the breast from extramammary carcinomas. APMIS 198189251–256. [DOI] [PubMed] [Google Scholar]
- 18.Lee S H, Park J M, Kook S H.et al Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med 200019257–262. [DOI] [PubMed] [Google Scholar]
- 19.Sneige N, Zachariah S, Fanning T V.et al Fine‐needle aspiration cytology of metastatic neoplasms in the breast. Am J Clin Pathol 19899227–35. [DOI] [PubMed] [Google Scholar]
- 20.Bohman L G, Bassett L W, Gold R H.et al Breast metastases from extramammary malignancies. Radiology 1982144309–312. [DOI] [PubMed] [Google Scholar]
- 21.Harris G C, Denley H E, Pinder S E.et al Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol 20032711–15. [DOI] [PubMed] [Google Scholar]
- 22.Lee A H S, Gillett C E, Ryder K.et al Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology 200648692–701. [DOI] [PubMed] [Google Scholar]
- 23.Badve S. Other malignant lesions of the breast. In: O'Malley FP, Pinder SE, eds. Breast pathology. Churchill Livingstone 2006283–301.
- 24.Chu P G, Weiss L M. Keratin expression in human tissues and neoplasms. Histopathology 200240403–439. [DOI] [PubMed] [Google Scholar]
- 25.Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer 200238758–763. [DOI] [PubMed] [Google Scholar]
- 26.Tot T. Patterns of distribution of cytokeratins 20 and 7 in special types of invasive breast carcinoma: a study of 123 cases. Ann Diagn Pathol 19993350–356. [DOI] [PubMed] [Google Scholar]
- 27.Abd El‐Rehim D M, Pinder S E, Paish C E.et al Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004203661–671. [DOI] [PubMed] [Google Scholar]
- 28.Pinkus G S, Kurtin P J. Epithelial membrane antigen‐a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol 198516929–940. [DOI] [PubMed] [Google Scholar]
- 29.Gillett C E, Bobrow L G, Millis R R. S100 protein in human mammary tissue‐immunoreactivity in breast carcinoma, including Paget's disease of the nipple, and value as a marker of myoepithelial cells. J Pathol 199016019–24. [DOI] [PubMed] [Google Scholar]
- 30.Dwarakanath S, Lee A K C, Delellis R A.et al S‐100 protein positivity in breast carcinomas: a potential pitfall in diagnostic immunohistochemistry. Hum Pathol 1987181144–1148. [DOI] [PubMed] [Google Scholar]
- 31.Robertson J F R, Ellis I O, Bell J.et al Carcinoembryonic antigen immunocytochemistry in primary breast cancer. Cancer 1989641638–1645. [DOI] [PubMed] [Google Scholar]
- 32.Zafrani B, Aubriot M H, Mouret E.et al High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology 200037536–545. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes A, Jasani B, Balaton A J.et al Frequency of oestrogen and progesterone receptor positivity by immunohistochemical analysis in 7016 breast carcinomas: correlation with patient age, assay sensitivity, threshold value, and mammographic screening. J Clin Pathol 200053688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadji M, Gomez‐Fernandez C, Ganjei‐Azar P.et al Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol 200512321–27. [DOI] [PubMed] [Google Scholar]
- 35.Dennis J L, Hvidsten T R, Wit E C.et al Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res 2005113766–3772. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann O, Deidesheimer T, Muehlenberg M.et al Immunohistochemical differentiation of metastatic breast carcinomas from metastatic adenocarcinomas of other common primary sites. Histopathology 199629233–240. [DOI] [PubMed] [Google Scholar]
- 37.Lau S K, Chu P G, Weiss L M. Immunohistochemical expression of estrogen receptor in pulmonary adenocarcinoma. Appl Immunohistochem Mol Morphol 20061483–87. [DOI] [PubMed] [Google Scholar]
- 38.Wick M R, Lillemoe T J, Copland G T.et al Gross cystic disease fluid protein‐15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha‐lactalbumin. Hum Pathol 198920281–287. [DOI] [PubMed] [Google Scholar]
- 39.Lagendijk J H, Mullink H, van Diest P J.et al Immunohistochemical differentiation between primary adenocarcinomas of the ovary and ovarian metastases of colonic and breast origin. Comparison between a statistical and an intuitive approach. J Clin Pathol 199952283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Recine M A, Deavers M T, Middleton L P.et al Serous carcinoma of the ovary and peritoneum with metastases to the breast and axillary lymph nodes: a potential pitfall. Am J Surg Pathol 2004281646–1651. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein N S, Bassi D, Uzieblo A. WT1 is an integral component of an antibody panel to distinguish pancreaticobiliary and some ovarian epithelial neoplasms. Am J Clin Pathol 2001116246–252. [DOI] [PubMed] [Google Scholar]
- 42.Lee B H, Hecht J L, Pinkus J L.et al WT1, estrogen receptor, and progesterone receptor as markers for breast or ovarian primary sites in metastatic adenocarcinoma to body fluids. Am J Clin Pathol 2002117745–750. [DOI] [PubMed] [Google Scholar]
- 43.Hwang H, Quenneville L, Yaziji H.et al Wilms tumor gene product: sensitive and contextually specific marker of serous carcinomas of ovarian surface epithelial origin. Appl Immunohistochem Mol Morphol 200412122–126. [DOI] [PubMed] [Google Scholar]
- 44.Tornos C, Soslow R, Chen S.et al Expression of WT1, CA 125, and GCDFP‐15 as useful markers in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol 2005291482–1489. [DOI] [PubMed] [Google Scholar]
- 45.Ordonez N G. Value of thyroid transcription factor‐1, E‐cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 200024598–606. [DOI] [PubMed] [Google Scholar]
- 46.Pettinato G, Manivel C J, Panico L.et al Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol 2004121857–866. [DOI] [PubMed] [Google Scholar]
- 47.Loy T S, Quesenberry J T, Sharp S C. Distribution of CA 125 in adenocarcinomas. An immunohistochemical study of 481 cases. Am J Clin Pathol 199298175–179. [DOI] [PubMed] [Google Scholar]
- 48.Frierson H F, Moskaluk C A, Powell S M.et al Large‐scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol 200334605–609. [DOI] [PubMed] [Google Scholar]
- 49.Ordonez N G. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol 2004271418–1428. [DOI] [PubMed] [Google Scholar]
- 50.Banerjee S S, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology 200036387–402. [DOI] [PubMed] [Google Scholar]
- 51.Miettinen M, Fernandez M, Franssila K.et al Microphthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma: comparison with four other melanoma markers. Am J Surg Pathol 200125205–211. [DOI] [PubMed] [Google Scholar]
- 52.Granter S R, Weilbaecher K N, Quigley C.et al Role for microphthalmia transcription factor in the diagnosis of metastatic malignant melanoma. Appl Immunohistochem Mol Morphol 20021047–51. [DOI] [PubMed] [Google Scholar]
- 53.Gajjar N A, Cochran A J, Binder S W. Is MAGE‐1 expression in metastatic malignant melanomas really helpful? Am J Surg Pathol 200428883–888. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann O, Georgi T, Dietel M. Utility of 123C3 monoclonal antibody against CD56 (NCAM) for the diagnosis of small cell carcinomas on paraffin sections. Hum Pathol 1997281373–1378. [DOI] [PubMed] [Google Scholar]
- 55.Kaufmann O, Dietel M. Expression of thyroid transcription factor‐1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology 200036415–420. [DOI] [PubMed] [Google Scholar]
- 56.Shin S J, DeLellis R A, Rosen P P. Small cell carcinoma of the breast‐additional immunohistochemical studies. Am J Surg Pathol 200125831–832. [DOI] [PubMed] [Google Scholar]
- 57.Shin S J, DeLellis R A, Ying L.et al Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol 2000241231–1238. [DOI] [PubMed] [Google Scholar]
- 58.Bejarano P A, Baughman R P, Biddinger P W.et al Surfactant proteins and thyroid transcription factor‐1 in pulmonary and breast carcinomas. Mod Pathol 19969445–452. [PubMed] [Google Scholar]
- 59.Rakha E A, Putti T C, Abd El‐Rehim D M.et al Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol 2006208495–506. [DOI] [PubMed] [Google Scholar]
- 60.Fulford L G, Easton D F, Reis‐Filhi J S.et al Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma. Histopathology 20064922–34. [DOI] [PubMed] [Google Scholar]
- 61.Livasy C A, Karaca G, Nanda R.et al Phenotypic evaluation of the basal‐like subtype of invasive breast carcinoma. Mod Pathol 200619264–271. [DOI] [PubMed] [Google Scholar]
- 62.Kaufmann O, Dietel M. Thyroid transcription factor‐1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology 2000368–16. [DOI] [PubMed] [Google Scholar]
- 63.Nadji M, Tabei S Z, Castro A.et al Prostatic origin of tumors. An immunohistochemical study. Am J Clin Pathol 198073735–739. [DOI] [PubMed] [Google Scholar]
- 64.Varma M, Morgan M, Jasani B.et al Polyclonal anti‐PSA is more sensitive but less specific than monoclonal anti‐PSA: implications for diagnostic prostatic pathology. Am J Clin Pathol 2002118202–207. [DOI] [PubMed] [Google Scholar]
- 65.Bassily N H, Vallorosi C J, Akdas G.et al Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol 2000113383–388. [DOI] [PubMed] [Google Scholar]
- 66.van Krieken J H J M. Prostate marker immunoreactivity in salivary gland neoplasms. A rare pitfall in immunohistochemistry. Am J Surg Pathol 199317410–414. [DOI] [PubMed] [Google Scholar]
- 67.DeYoung B R, Wick M R. Immunohistologic evaluation of metastatic carcinomas of unknown origin: an algorithmic approach. Semin Diagn Pathol 200017184–193. [PubMed] [Google Scholar]
- 68.Gatalica Z, Norris B A, Kovatich A J. Immunohistochemical localization of prostate‐specific antigen in ductal epithelium of male breast. Potential diagnostic pitfall in patients with gynecomastia. Appl Immunohistochem Mol Morphol 20008158–161. [DOI] [PubMed] [Google Scholar]
- 69.Kidwai N, Gong Y, Sun X.et al Expression of androgen receptor and prostate‐specific antigen in male breast carcinoma. Breast Cancer Res 20046R18–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carder P J, Speirs V, Ramsdale J.et al Expression of prostate specific antigen in male breast cancer. J Clin Pathol 20055869–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldstein N S, Long A, Kuan S F.et al Colon signet ring cell adenocarcinoma: immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol 20008183–188. [DOI] [PubMed] [Google Scholar]
- 72.van Velthuysen M L F, Taal B G, van der Hoeven J J M.et al Expression of oestrogen receptor and loss of E‐cadherin are diagnostic for gastric metastasis of breast carcinoma. Histopathology 200546153–157. [DOI] [PubMed] [Google Scholar]
- 73.O'Connell F P, Wang H H, Odze R D. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med 2005129338–347. [DOI] [PubMed] [Google Scholar]
- 74.Moskaluk C A, Zhang H, Powell S M.et al Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 200316913–919. [DOI] [PubMed] [Google Scholar]
- 75.Werling R W, Yaziji H, Bacchi C E.et al CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 200327303–310. [DOI] [PubMed] [Google Scholar]
- 76.Renshaw A A, Richie J P. Subtypes of renal cell carcinoma. Different onset and sites of metastatic disease. Am J Clin Pathol 1999111539–543. [DOI] [PubMed] [Google Scholar]
- 77.McGregor D K, Khurana K K, Cao C.et al Diagnosing primary and metastatic renal cell carcinoma: the use of the monoclonal antibody ‘Renal Cell Carcinoma Marker'. Am J Surg Pathol 2001251485–1492. [DOI] [PubMed] [Google Scholar]
- 78.Iwaya K, Ogawa H, Izumi M.et al Stromal expression of CD10 in invasive breast carcinoma: a new predictor of clinical outcome. Virchows Arch 2002440589–593. [DOI] [PubMed] [Google Scholar]
- 79.Chu P, Arber D A. Paraffin‐section detection of CD10 in 505 nonhematopoietic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol 2000113374–382. [DOI] [PubMed] [Google Scholar]
- 80.Langner C, Ratschek M, Rehak P.et al Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol 2004171611–614. [DOI] [PubMed] [Google Scholar]
- 81.Kim M K, Kim S. Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol 200210332–338. [DOI] [PubMed] [Google Scholar]
- 82.Cai Y C, Banner B, Glickman J.et al Cytokeratin 7 and 20 and thyroid transcription factor 1 can help distinguish pulmonary from gastrointestinal carcinoid and pancreatic endocrine tumors. Hum Pathol 2001321087–1093. [DOI] [PubMed] [Google Scholar]
- 83.Sapino A, Righi L, Cassoni P.et al Expression of apocrine differentiation markers in neuroendocrine breast carcinomas of aged women. Mod Pathol 200114768–776. [DOI] [PubMed] [Google Scholar]
- 84.Viale G, Doglioni C, Gambacorta M.et al Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 1992702268–2277. [DOI] [PubMed] [Google Scholar]
- 85.Dunne B, Lee A H S, Pinder S E.et al An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol 2003341009–1015. [DOI] [PubMed] [Google Scholar]
- 86.Hugh J C, Jackson F I, Hanson J.et al Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer 1990662602–2611. [DOI] [PubMed] [Google Scholar]
- 87.Domchek S M, Hecht J L, Fleming M D.et al Lymphomas of the breast: primary and secondary involvement. Cancer 2002946–13. [DOI] [PubMed] [Google Scholar]
- 88.Duncan V E, Reddy V V B, Jhala N C.et al Non‐Hodgkin's lymphoma of the breast: a review of 18 primary and secondary cases. Ann Diagn Pathol 200610144–148. [DOI] [PubMed] [Google Scholar]
- 89.Aguilera N S I, Tavassoli F A, Chu W S.et al T‐cell lymphoma presenting in the breast: a histologic, immunophenotypic and molecular genetic study of four cases. Mod Pathol 200013599–605. [DOI] [PubMed] [Google Scholar]
- 90.Shanks J H, Banerjee S S. VS38 immunostaining in melanocytic lesions. J Clin Pathol 199649205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banerjee S S, Shanks J H, Hasleton P S. VS38 immunostaining in neuroendocrine tumours. Histopathology 199730256–259. [DOI] [PubMed] [Google Scholar]
- 92.Kambham N, Kong C, Longacre T A.et al Utility of syndecan‐1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: a tissue microarray study of 1,754 cases. Appl Immunohistochem Mol Morphol 200513304–310. [DOI] [PubMed] [Google Scholar]
- 93.Austen J.Emma. London: Penguin, 1994