Abstract
An atypical case of sporadic Creutzfeldt–Jakob disease (CJD) is described in a 78‐year‐old woman homozygous for methionine at codon 129 of the prion protein (PrP) gene. The neuropathological signature was the presence of PrP immunoreactive plaque‐like deposits in the cerebral cortex, striatum and thalamus. Western blot analysis showed a profile of the pathological form of PrP (PrPSc) previously unrecognised in sporadic CJD, marked by the absence of diglycosylated protease resistant species. These features define a novel neuropathological and molecular CJD phenotype.
Prion diseases are a group of fatal neurodegenerative disorders that share a similar pathogenic mechanism (ie, conversion of the normal cellular form of the host encoded prion protein (PrPC) to abnormal, disease specific isoforms (termed PrPSc)).1 A remarkable feature of prion diseases is their heterogeneity in phenotypic expression that in sporadic Creutzfeldt–Jakob disease (sCJD) has been related to the methionine/valine polymorphism at codon 129 of the prion protein gene (PRNP) and to the physicochemical characteristics of PrPSc. At least two types of PrPSc have been found in sCJD, that are distinguished by the size of the protease resistant core. On these grounds, different sCJD phenotypes have been identified.2,3 Here we report a novel sCJD phenotype, marked by a previously unrecognised association of PrPSc type and neuropathological profile.
Methods
The patient was investigated following a diagnostic protocol, including CSF examination, electroencephalographic recordings and standard MRI of the brain. The complete sequence of the PRNP open reading frame, including the region of signal peptide, was carried out as described previously.4
The neuropathological study was performed on Carnoy and formalin fixed sections of the brain, stained with haematoxylin–eosin, cresyl violet for Nissl substance, Heidenhain–Woelcke for myelin, thioflavine S for amyloid, Bodian and Gallyas silver stains and immunohistochemistry with antibodies against Aβ (pan‐β, 1:1000; Biosource, Camarillo, California, USA), phosphorylated tau protein (AT8, 1:200; Innogenetics, Gent, Belgium), α‐synuclein (clone 4D6, 1:10000; Signet, Dedham, Massachusetts, USA) and prion protein. The latter included the monoclonal antibodies 3F4 (epitope at residues 109–112 of human PrP, 1:800; DakoCytomation, Glostrup, Denmark), 6H4 (epitope at residues 144–152, 1:500; Prionics, Zurich, Switzerland) and SP214 (epitope at residues 214–231,5 1:200). For Aβ and α‐synuclein, sections were pretreated with formic acid (98%, 15 min), while for PrP immunohistochemistry, sections were pretreated as previously reported.6 The immunoreaction was visualised using the EnVision Plus/Horseradish Peroxidase system (DakoCytomation) and 3–3′‐diaminobenzidine.
Western blot analysis was carried out on samples of several areas of the cerebral cortex, subcortical nuclei and cerebellum, using the above mentioned anti‐PrP antibodies, as previously described.4 The analysis was carried out prior to and after PK digestion on sample aliquots containing 100 μg of protein. To enhance PrPSc detection, the study was also carried out on samples obtained by phosphotungstic acid precipitation of 50–200 μl of 10% homogenate.7
Results
Clinical and laboratory findings
A 78‐year‐old woman, affected since the age of 74 by parkinsonism unresponsive to DOPA treatment, developed a rapid decline in motor and cognitive performances, with confusional state, dysphasia, insomnia and urinary incontinence. About 1 month after the onset of these symptoms, she was found unconscious in bed one morning and taken to the hospital. On admission, she showed a decorticate rigidity with flexed arms and extended legs, myotic reactive pupils and continuous and diffuse myoclonic jerks of her head and limbs. CSF examinations demonstrated the presence of 14.3.3 and high levels of tau protein (7000 pg/ml; normal 66–276). The patient was homozygous for methionine at codon 129 of the PRNP gene, and no mutations were found.
Several electroencephalographic recordings showed an initial pattern characterised by slow biphasic and triphasic periodic waves (synchronous with myoclonic jerks) evolving towards a more slow, low amplitude, non‐reactive background activity in the end stages of the disease. Cerebral MRI revealed diffuse symmetrical cortical and subcortical atrophy without signal abnormalities in the basal ganglia. A remarkable hyperintensity in T2 weighted images in bilateral deep white matter extended to the subcortical parietal and temporal lobes, without enhancement after administration of paramagnetic substances (fig 1A, B). Both atrophy and signal abnormalities progressed during the course of the disease. The patient had a prolonged partial adversive seizure with involvement of the face and right arm, reverted by treatment with phenobarbital and phenytoin. She died 6 weeks after admission. An autopsy was performed.
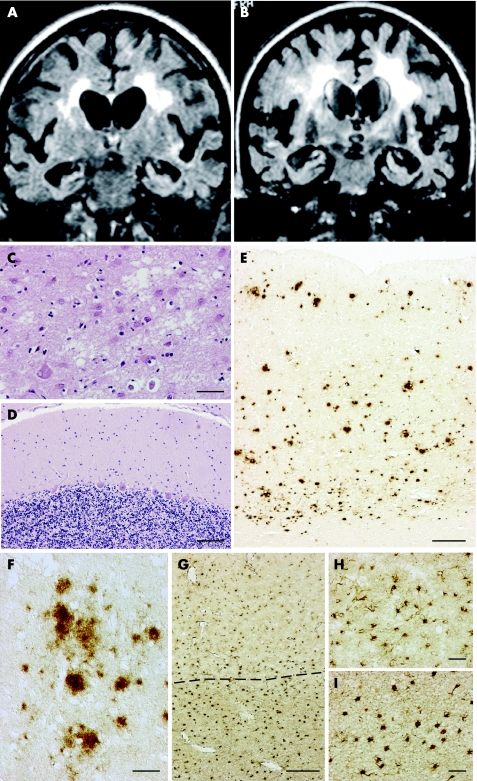
Figure 1 MRI and neuropathological findings. Cortical and subcortical atrophy with ventricular enlargement was already evident at the first examination (A, MRI at admission) and was more severe 2 weeks before death (B). (A) and (B) are fluid attenuated inversion recovery image sequences and revealed progressive diffuse signal hyperintensity in the white matter of the centrum ovale, while signal abnormalities were not detected in the basal ganglia. The neuropathological analysis showed the presence of focal, subtle spongiosis in the cerebral cortex (C, frontal cortex, haematoxylin–eosin) and a normal histological picture in the cerebellum (D, haematoxylin‐eosin). The immunohistochemical pattern of prion protein deposition is defined by numerous plaque‐like deposits that are distributed in the cortical thickness (E). They appear as diffuse amorphous deposits and are round irregularly shaped or multicentric, up to 100 μm in diameter, sometimes encompassing unstained cell bodies (F). Reactive gliosis (G–I, immunohistochemistry with antiglial fibrillary acidic protein) severely affected both the cerebral cortex (G, area above the dotted line; higher magnification in H) and the subcortical white matter (G, area below the dotted line; higher magnification in I). Scale bars: (C) 25 μm; (D) 100 μm; (E) 400 μm; (F) 50 μm; (G) 250 μm; (H, I) 50 μm.
Neuropathology
The supratentorial structures were atrophic with symmetric enlargement of the lateral ventricles (fresh brain weight 1030 g), with cortical nerve cell loss and gliosis more severe in the frontal and temporal areas, where occasional foci of spongiosis were observed (fig 1C). In these lobes, the subcortical white matter showed rarefaction of myelin and marked gliosis (fig 1G–I), in the absence of ischaemic or hypoxic changes. No significant vascular lesions were present, in particular no hyaline changes in the vessel walls. The cerebellum and brainstem were free of significant pathology (fig 1D).
In contrast with the paucity of spongiosis, PrP immunoreactivity was consistent and almost exclusively in the form of plaque‐like deposits, intensely labelled by all anti‐PrP antibodies used (fig 1E) and abundant in the cerebral cortex, striatum and thalamus, while absent in the cerebellum and brainstem. These PrP focal deposits were round, irregularly shaped or multicentric, up to 100 μm in diameter, sometimes encompassing unstained cell bodies (fig 1F); they were not associated with dystrophic neurites or immunolabelled by anti‐Aβ antibodies, and did not exhibit the tinctorial and optical properties of amyloid. The diffuse (“synaptic‐type”) finely granular PrP immunostaining was faint and confined to a few areas of the cerebral cortex.
In the mesial temporal structures, numerous argyrophilic, tau‐positive granular or spindle shaped profiles scattered in the neuropil and occasional neurofibrillary tangles were seen, suggesting the presence of argyrophilic grain disease. Aβ protein deposits, Lewy bodies or α‐synuclein positive inclusions were absent. Phospho‐tau immunoreactive perikarya and neurites were also observed in the substantia nigra and locus coeruleus.
Biochemical analysis
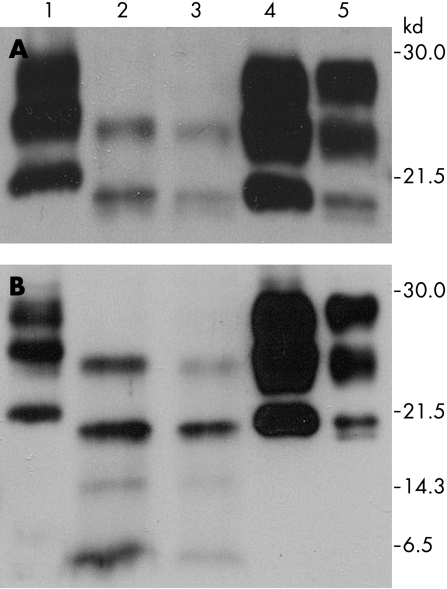
Western blot analysis of brain homogenates with the antibody 3F4 without PK digestion showed a typical three band pattern with di, mono and non‐glycosylated PrP isoforms. In contrast, analysis after PK digestion revealed only two PrPSc bands corresponding to monoglycosylated (24 kDa) and unglycosylated (19 kDa) PrPSc species, while the diglycosylated band was absent. The non‐glycosylated PrPSc isoform had the same electrophoretic mobility of type 2 PrPSc by Parchi and colleagues2 (fig 2).
Figure 2 Western blot analysis of cingulum and parietal cortex (lanes 2 and 3) performed with 3F4 (A) and SP214 (B) antibody after PK digestion showed the presence of two bands of 24 and 19 kDa and the absence of the diglycosylated pathological form of the prion protein (PrPSc) species. Two additional 14 and 6 kDa fragments of PrPSc were detected with SP214 antibody. This profile is different from type I (lane 1), type IIA (lane 4) and type IIB (lane 5) PrPSc.
Using the SP214 antibody that recognises a C terminal epitope of PrP, two additional 14 and 6 kDa fragments of PrPSc were detected, suggesting a further cleavage producing protease resistant C terminal fragments of the prion protein (fig 2).
Discussion
In transmissible spongiform encephalopathies, prion strains are associated with distinct PrPSc types that can be distinguished by western blot analysis on the basis of different cleavage sites to proteinase K and different glycoform ratios.8 Phenotypic heterogeneity in sCJD is well documented, and systematic classifications of the disease phenotypes have been proposed2,3 that include subtypes of sCJD resulting from the combination of the three possible genotypes at codon 129 of PRNP and the different biochemical types of PrPSc.
In our institution, as the Reference Centre for Prion Diseases of Regione Lombardia, we have examined more than 200 cases of CJD since 1996 and could classify them according to the scheme proposed by Parchi and colleagues2 on the basis of the neuropathological, immunohistochemical and biochemical analyses. By contrast, the patient that we report here does not fit any category of Parchi's classification of sCJD2 nor others.3 The presence of the mono and unglycosylated PrPSc fractions and the absence of the diglycosylated fraction in our case constitutes an original two band PrPSc biochemical profile clearly different from the classical three band PrPSc pattern, extending the range of possible PrPSc variations in sCJD. Moreover, the finding of small sized C terminal PrPSc fragments (14 and 6 kDa), slightly different from those previously reported in sCJD,9 contributes to the peculiarity of the biochemical profile. Different ratios of the three PrPSc glycoforms have been reported in association with PRNP mutations, such as E200K, but total absence of the diglycosylated PrPSc band has been described only in patients carrying the mutations T183A and V180I that are in close proximity to one of the two N‐glycosylation sites of PrPC.10,11
The neuropathological features and the PrP immunostaining pattern of our patient were also unusual in themselves, and especially if considered in combination with the biochemical and genetic features. The peculiarity consists in the scarceness of spongiosis and of the synaptic PrP immunoreactivity. Abnormal PrP accumulation was almost exclusively of the form of focal deposits, appearing quite different from those found in sCJD patients homozygous for methionine at codon 129 of PRNP with type 2 PrPSc that are typically perivacuolar.12 Moreover, the PrP deposits of our patient are not like the florid plaques of variant CJD,13 the uni‐ or multicentric PrP plaques of Gerstmann–Sträussler–Scheinker disease14 or the focal PrP deposits found in familial CJD with large insertional PRNP mutations.15
From the clinical standpoint, the patient history is to a large extent in compliance with the diagnosis of CJD; however, peculiar findings are represented by the lack of MRI signal alterations in the basal ganglia and by the presence of severe cerebral atrophy and white matter abnormalities. Moreover, prior to the rapid final cognitive and neurological decline, a 4 year history of parkinsonism occurred, most likely being the consequence of the co‐occurrence of argyrophilic grain disease.
In conclusion, our results demonstrate the existence of further rare molecular subtypes of sCJD, whose characterisation may provide clues for the elucidation of the relation between biochemical characteristics of PrPSc and clinicopathological features of this disorder.
Acknowledgements
Supported by the Italian Ministry of Health and the European Community (LSHM‐CT‐2004‐503039 and Neuroprion FOOD‐CT‐2004‐506579). We are grateful to Dr Blas Frangione and Dr Frances Prelli (New York University, New York, USA) for providing the SP214 antibody.
Abbreviations
PRNP - prion protein gene
PrP - prion protein
sCJD - sporadic Creutzfeldt–Jakob disease
Footnotes
Competing interests: None.
References
- 1.Prusiner S B. Prions. Proc Natl Acad Sci U S A 19989513363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parchi P, Giese A, Capellari S.et al Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 199946224–233. [PubMed] [Google Scholar]
- 3.Hill A F, Joiner S, Wadsworth J D.et al Molecular classification of sporadic Creutzfeldt–Jakob disease. Brain 20031261333–1346. [DOI] [PubMed] [Google Scholar]
- 4.Rossi G, Giaccone G, Giampaolo L.et al Creutzfeldt–Jakob disease with a novel four extra‐repeat insertional mutation in the PrP gene. Neurology 200055405–410. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez‐Huete A, Lievens P M, Vidal R.et al Endogenous proteolytic cleavage of normal and disease‐associated isoforms of the human prion protein in neural and non‐neural tissues. Am J Pathol 19981531561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giaccone G, Canciani B, Puoti G.et al Creutzfeldt–Jakob disease: Carnoy's fixative improves the immunohistochemistry of the proteinase K‐resistant prion protein. Brain Pathol 20001031–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadsworth J D F, Jonier S, Hill A F.et al Tissue distribution of protease resistant prion protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet 2001358171–180. [DOI] [PubMed] [Google Scholar]
- 8.Bruce M E, McConnell I, Fraser H.et al The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implication for the nature of the agent and host control of pathogenesis. J Gen Virol 199172595–603. [DOI] [PubMed] [Google Scholar]
- 9.Zanusso G, Farinazzo A, Prelli F.et al Identification of distinct N‐terminal truncated forms of prion protein in different Creutzfeldt–Jakob disease subtypes. J Biol Chem 200427938936–38942. [DOI] [PubMed] [Google Scholar]
- 10.Chasseigneaux S, Haik S, Laffont‐Proust I.et al V180I mutation of the prion protein gene associated with atypical PrPSc glycosylation. Neurosci Lett 2006408165–169. [DOI] [PubMed] [Google Scholar]
- 11.Capellari S, Zaidi S I, Long A C.et al Thr183Ala mutation, not the loss of the first glycosylation site, alters the physical properties of the prion protein. J Alzeimers Dis 2000227–35. [DOI] [PubMed] [Google Scholar]
- 12.Budka H, Head M W, Ironside J W.et al Sporadic Creutzfeldt–Jakob disease. In: Dickson D, ed. Neurodegeneration: the molecular pathology of dementia and movement disorders. Basel, Switzerland: ISN Neuropath Press, 2003287–297.
- 13.Will R G, Ironside J W, Zeidler M.et al A new variant of Creutzfeldt–Jakob disease in the UK. Lancet 1996347921–925. [DOI] [PubMed] [Google Scholar]
- 14.Ghetti B, Dlouhy S R, Giaccone G.et al Gerstmann–Sträussler–Scheinker disease. Brain Pathol 1995561–75. [DOI] [PubMed] [Google Scholar]
- 15.Parchi P, Capellari S, Chen S G.et al Familial Creutzfeldt–Jakob disease. In: Dickson D, ed. Neurodegeneration: the molecular pathology of dementia and movement disorders. Basel, Switzerland: ISN Neuropath Press, 2003298–306.